One of the major barriers to Tuberculosis vaccine development has been the lack of a correlate of protection or biomarker that can aid in the selection of vaccine candidates at early stages of the vaccine pipeline. Mycobacterial Growth Inhibition Assays (MGIAs) come as an alternative, especially for the functional analysis of blood and cell samples, post-vaccination, to inhibit the growth of Mycobacterium Tuberculosis (Mtb) in vitro.
In this study, Tanner et al., sought to optimise and harmonise a direct MGIA using cryopreserved peripheral blood mononuclear cells (PBMCs) and compare results across three different laboratories to assess precision and reproducibility of the test for clinical trial use.
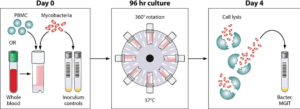
PBMCs were cultured together with BCG Pasteur for 96 hours in sealed rotating screw-cap tubes as well as static 48-well plates. At the end of the culture period, cells were lysed using different agents while other cells were not lysed at all. After centrifugation, the pellet was transferred to BACTEC MGIT tubes and the BACTEC system used to carry out mycobacterial quantification.
IFN-γ production, measured using the ELISA method, and cell viability after the 96 hour period were observed to significantly increase when cells were co-cultured in tissue culture plates in comparison to the use of screw-cap tubes. Cell viability, however, did not correlate with control of mycobacterial growth. There was also no correlation between the amount of IFN-γ present and control of mycobacterial growth. Tanner et al., believe that this suggests that the assay may be used to measure IFN-γ-independent responses, thereby supporting its use as an additional tool for measuring vaccine immunogenicity.
As part of assay harmonisation, mycobacterial growth at different sites was compared between naïve and vaccinated volunteers using both screw-cap tubes and 48-well plates. There was no significant difference noted in MGIA results showing that consistent and comparable results can be obtained if this assay were to be used at different vaccine trial sites. The next step for this assay is to see whether control of mycobacterial growth correlates with in vivo protection and to later on test the assay on TB vaccine candidates.
Journal Article: Rachel Tanner et al., 2018. Optimisation, harmonisation and standardisation of the direct mycobacterial growth inhibition assay using cryopreserved human peripheral blood mononuclear cells. Journal of Immunological Methods
Article by Vanessa Mwebaza Muwanga