Alzheimer’s disease is characterized by the build-up of a protein called amyloid beta, which forms plaques in the brain. These plaques trigger a cascade of events leading to brain degeneration and cognitive decline. New Alzheimer’s drugs work by tagging amyloid beta for removal by immune cells, but researchers have discovered a promising alternative approach. This study explores a different strategy – directly stimulating the brain’s natural immune soldiers, microglia, to eliminate the harmful plaques (Figure 1). The researchers used an antibody to activate microglia in mice with Alzheimer’s-like disease. This approach successfully reduced amyloid beta plaques in the brain and improved behavioral abnormalities in the mice.
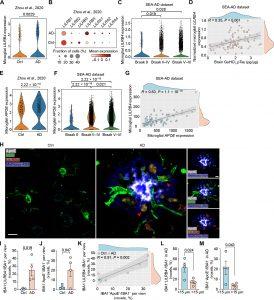
Figure 1: Microglial LILRB4 expression is correlated with AD pathology.
(A) Violin plot of microglial expression of LILRB4 in brain tissue from patients with AD and from control (Ctrl) participants. n = 476 microglia from 11 Ctrl and 424 microglia from 11 AD brain specimens. A previously published human snRNA-seq dataset was used for this analysis (36). (B) Dot plot of microglial expression of LILRs in brain tissue from patients with AD and Ctrl participants. Same snRNAseq dataset as in (A). (C) Microglial expression of LILRB4 in brain tissue from patients with different Braak scores. n = 84 patients: 2 Braak 0 (208 microglia); 4 Braak II (739 microglia); 6 Braak III (566 microglia); 23 Braak IV (2617 microglia); 34 Braak V (3598 microglia); 15 Braak VI (1664 microglia). The SEA-AD dataset was used for this analysis. (D) Spearman’s correlation of microglial expression of LILRB4 with pTau in GuHCl fraction of AD brain lysates. n = 84 participants. Same dataset as in (C). (E) Microglial expression of APOE in brain tissue from patients with AD and Ctrl participants. n = 11 cases in both groups. Same snRNAseq dataset as in (A). (F) Microglial expression of APOE in brain tissue from patients with different Braak scores. Same dataset as in (C). (G) Spearman’s correlation of microglial LILRB4 expression with microglial APOE expression. n = 84 participants. Same dataset as in (C). (H) Representative immunofluorescence images for IBA1 (green), LILRB4 (red), ApoE (white), and amyloid plaques marker methoxy-X04 (blue) in the dorsolateral prefrontal cortex of participants with AD and Ctrl. Scale bar, 30 μm in the all-channel and channel-separated images. Red arrows point to ApoE+LILRB4+IBA1+ microglia. (I and J) Quantification of LILRB4+ (I) and ApoE+ (J) voxels per IBA+ voxels per view of the image. n = 5 to 8 views of image per case were quantified. Four Ctrl (23 images) and AD (25 images) cases were analyzed. (K) Spearman’s correlation of LILRB4 with APOE in microglia. n = 4 Ctrl and 4 AD cases from (I) and (J). (L) Quantification of the percentage of IBA1+LILRB4+ voxels colocalized with IBA1+ voxels in four AD cases within or beyond 15 μm around Aβ plaques. A total of 25 plaques in four AD cases were analyzed. (M) Quantification of the percentage of IBA1+APOE+ voxels colocalized with IBA1+ voxels in four AD cases within or beyond 15 μm around Aβ plaques. A total of 25 plaques in four AD cases were analyzed. In all graphs, data are presented as means ± SEM. Significance was determined using the Wilcoxon test (A, C, E, and F) or a Student’s t test (I, J, L, and M).
The exciting implication is that this approach might extend beyond Alzheimer’s. Many neurodegenerative diseases, such as Parkinson’s and ALS, are also characterized by toxic protein buildup in the brain. Researchers are enthusiastic about exploring this immunotherapy strategy to target and eliminate these harmful proteins in other diseases.
Microglia normally surround plaques, acting as a barrier to control the spread of damaging proteins. They also can engulf and destroy these plaques. However, in Alzheimer’s disease, microglia become somewhat apathetic. The study suggests that a protein called APOE, found in amyloid plaques, might be responsible for this passivity. APOE binds to a receptor (LILRB4) on microglia, essentially inactivating them.
The researchers observed that in mice and humans with Alzheimer’s, microglia surrounding plaques produce and position LILRB4, making them less effective in clearing amyloid beta. To address this, they developed an antibody that blocks APOE from binding to LILRB4. This approach successfully restored microglia’s ability to engulf and clear amyloid beta plaques in mice.
Interestingly, clearing amyloid beta plaques in mice also improved their risk-taking behaviour, which can be a significant issue for Alzheimer’s patients. This suggests that this approach might not only slow disease progression but also improve cognitive function. Future studies will examine the antibody’s effect on tau tangles, another hallmark of Alzheimer’s that appears later in the disease.
Some existing Alzheimer’s drugs that target amyloid beta can cause swelling and bleeding in the brain. This study used mice without amyloid beta build-up in blood vessels, so the safety of this approach in regard to blood vessel plaques remains unclear.
Lecanemab, the first approved drug proven to modify the course of Alzheimer’s, validates the importance of targeting amyloid beta. This new research on microglia activation offers a fresh perspective and opens doors for developing new immunotherapies with potentially improved safety profiles.
Journal article: Jinchao Hou, J., et al., 2024. Antibody-mediated targeting of human microglial leukocyte Ig-like receptor B4 attenuates amyloid pathology in a mouse model. Science Translational Medicine.
Summary by Stefan Botha










