Mitochondria possess their own genetic code or DNA and can almost be described as a cell within a cell as they are self-contained organelles. They are commonly referred to as the powerhouse of the cell, providing chemical energy for all things living.
During moments of stress or if damaged, mitochondria release mitochondrial DNA (mtDNA) into the periphery which can result in inflammation in the body. Individuals with autoimmune conditions such as rheumatoid arthritis have been shown to have elevated levels of circulating oxidised mtDNA. Some questions remain, is mtDNA an indicator of disease pathology and what does it play in this pathology.
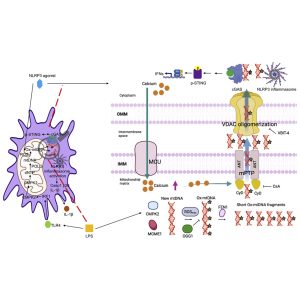
In a recent paper, researchers have identified and described a biochemical pathway that is essential for the production, transport, and action of oxidised mtDNA which may trigger inflammation in the body (Figure 1). Targeting this pathway may lead to the mitigation of inflammatory events experienced in individuals with autoimmune conditions. (READ MORE).
The enzyme FEN1 is responsible for the ‘chopping’ of mtDNA into small fragments so that it can be expelled by mitochondria following the uptake of calcium ions from the cytosol which generates reactive oxygen species (ROS). ROSs results in the oxidation of mtDNA and causes pore formation in mitochondrial membranes through which mtDNA pass. All of this is as a response to infection or tissue damage.
Following this process, expelled and oxidised mtDNA fragments bind to NLRP3 and cGAS which are key role players in the inflammatory response. This plays a significant role in autoimmune diseases whereby the immune system attacks healthy cells of the individual.
These findings may open up the field to research into inhibitors of these process and enzymes in the hope to combat autoimmune disease.
Journal article: Xian, H., et al., 2022. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity.
Summary by Stefan Botha