Microglia help keep the brain in balance by cleaning up debris, fighting infection, and responding to injury. However, in Alzheimer’s, they can become overactive, contributing to harmful inflammation.
In a breakthrough that could reshape how Alzheimer’s is treated, researchers have discovered that enhancing a natural calming signal in the brain’s immune system may prevent or reduce the inflammation that drives neurodegeneration in Alzheimer’s disease (AD) (Figure 1). The study spotlights the brain chemical norepinephrine and its interaction with microglia—the immune cells of the brain—as a key mechanism that could be targeted to slow disease progression.
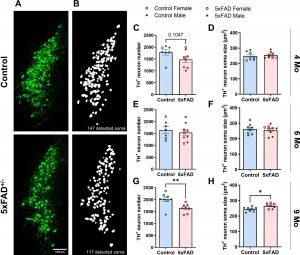
Figure 1: Loss of NE neurons in the LC in 5xFAD mice at advanced amyloid pathology stage. (A) Representative 20x confocal images of LC neurons immunolabeled with TH (scale bar = 100 μm). (B) Representative soma detection using Cellpose custom-trained model. (C-H) Number and size of LC neurons are comparable between control and 5xFAD mice at 4 months (C-D) and 6 months (E-F), LC neuron number is lower while neuronal size is larger at 9 months in 5xFAD compared to control mice (G-H). n = 7–9 mice per genotype per age group. Student’s t-test; *p < 0.05, **p < 0.01.
This study reveals how norepinephrine, a hormone and neurotransmitter best known for its role in stress responses, acts as a natural anti-inflammatory signal for microglia.
The calming effect of norepinephrine is mediated by a receptor on microglia called β2-adrenergic receptor (β2AR). In healthy brains, this receptor helps regulate inflammation. But in Alzheimer’s, especially around amyloid plaques—the hallmark protein clumps of the disease—these receptors decline, making microglia more likely to fuel damaging inflammation.
Using a mouse model of Alzheimer’s, the team explored what happens when the β2AR receptor is blocked or stimulated:
- Blocking β2AR worsened disease markers: more amyloid plaques, higher inflammation, and more nerve damage.
- Activating β2AR had the opposite effect: inflammation dropped, and neuronal damage was reduced.
The research highlights the importance of early intervention, and supports the idea that Alzheimer’s is not just a disease of neurons but also of the immune system’s failure to regulate itself.
Journal article: L.H.D. Le., et al, 2025. Noradrenergic signaling controls Alzheimer’s disease pathology via activation of microglial β2 adrenergic receptors, Brain, Behavior, and Immunity.
Summary by Stefan Botha










