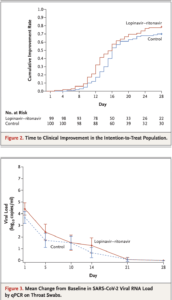
Source: Bars in Figure 3 indicate 95% confidence intervals. Results less than the lower limit of quantification of polymerase-chain-reaction (PCR) assay and greater than the limit of qualitative detection are imputed with 1 log10 copies per milliliter; results for patients with viral-negative RNA are imputed with 0 log10 copies per milliliter. Among the 199 patients, 130 (59 patients in the lopinavir–ritonavir group and 71 in the standard-care group) had virologic data that were used for viral load calculation, whereas the rest of the patients had undetectable viral RNA on throat swabs over the time. (Source: Cao et al., 2020 NEJM)
Results of a randomised, controlled, open-label trial involving hospitalised adult patients with confirmed SARS-CoV-2 infection were reported in a recent article in the New England Journal of Medicine. Patients were randomly assigned to receive either lopinavir–ritonavir twice a day for 14 days in addition to standard care, or standard care alone. The primary end point was the time to clinical improvement. Laboratory-confirmed SARS-CoV-2 infected patients (n=199) underwent randomisation with 99 assigned to lopinavir–ritonavir and 100 to the standard-care group. Treatment with lopinavir–ritonavir did not improve time to clinical improvement; did not improve mortality and the proportion of patients with detectable viral RNA at various time points were similar to the standard of care group of patients. Lopinavir–ritonavir treatment was stopped early in 13 patients (13.8%) because of gastrointestinal adverse events. The authors conclude that “we found that lopinavir–ritonavir treatment did not significantly accelerate clinical improvement, reduce mortality, or diminish throat viral RNA detectability in patients with serious Covid-19.”
Journal Article: Cao et al., 2020. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. NEJM
Article by Clive Gray










