BCG vaccination, that is still the primary vaccine given for prevention of tuberculosis (TB) has been studied widely in very many contexts outside of just how the immune system responds to Mycobacterium tuberculosis. As a result of these great efforts, we know now that BCG can provide non-specific protection from respiratory tract infections (RTIs) and this results from BCG being able to train the immune system through various mechanisms such as epigenetic reprogramming.
Through a double blind, randomised, controlled, phase 3 trial, carried out in health care workers in South Africa, Upton and colleagues sought to find out whether BCG brought about a reduction in morbidity and mortality as a result of COVID-19.
In the year 2020, 1000 healthcare workers were recruited at three different locations across the Western Cape i.e., TASK Cape Town, TASK Eden, and University of Cape Town Lung Institute (UCTLI). Using a computer-based program, participants were randomly assigned 1:1 to receive an intradermal injection of either 0.1ml of the reconstituted BCG vaccine (BCG-Vaccin Statens Serum Institut, Danish strain 1331) or 0.1ml of placebo composed of 0.9% saline solution. Participants were followed-up for a period of 52 weeks to find out about any adverse effects as well get information on their exposure to COVID.
From the results the authors came to a conclusion that BCG did not confer any protection from SARS-CoV-2 infection or other related severe COVID-19 disease in healthcare workers that were part of this study. There have been studies that have been for and against this. One other study, highlighted here did also conclude that there was no evidence that BCG vaccination can protect against sars cov 2 infection .
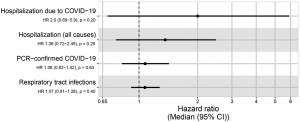
Figure 1: Figure 1: “Forest plots for the effect in the BCG group compared to placebo (reference) on COVID-19 hospitalisation, all-cause hospitalisation, PCR confirmed COVID-19, and RTI. Represented as a median hazard ratio (circle) and 95% confidence interval (whiskers). COVID-19 = coronavirus disease 2019, PCR = polymerase chain reaction, HR = hazard ratio, CI = confidence interval (Upton et al, 2022).
As hinted on initially, there have been some studies done that do indicate the presence of non-specific protection conferred by BCG with previous results reporting significant reductions in RTIs across different age groups. Since the results from this trial do not exactly agree with these previous studies that have demonstrated otherwise, the authors did try to think about what could account for this. One working theory that came up was the possibility of different population and immunological factors driving this phenomenon.
This study took part in South Africa, one country that is highly endemic for TB and thus over half, if not more of the healthcare workers recruited were latently infected. There is a possibility that latent TB infection could have blocked or masked the non-specific effects of BCG.
Based on what was seen from this trial, this is what Upton and colleagues had to say when it comes to the implications of these results, “Non-specific immune effects of BCG revaccination may be population-, age-, pathogen- or disease-specific. We recommend that BCG not be used for the prevention or mitigation of COVID-19 outside of a clinical trial, at least until results of other trials underway in different settings are known.”
Journal article: Upton, C.M., et al., 2022. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: A double-blind, randomised, controlled, phase 3 trial. eClinicalMedicine.
Summary by Vanessa Muwanga










