Disclaimer: This is a summary of an article that is a preprint and has not been peer reviewed.
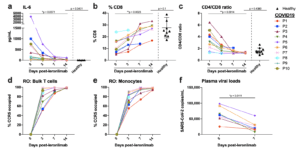
Figure 2. Reversal of immune dysfunction and CCR5 receptor occupancy in critically ill COVID-19 patients after leronlimab administration. a-c, Plasma levels of IL-6 (a), and peripheral blood CD8+ T cell percentages of CD3+ cells (b) and CD4/CD8 T cell ratio (c) at days 0 (n=10), 3 (n=10), 7 (n=7), and 14 (n=6) post-leronlimab administration. Healthy controls (n=10) shown in black triangles. Graphs display p-values calculated by Dunn’s Kruskal-Wallis test: not significant p > 0.05, *p ≤ 0.05, ** p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. d-e, CCR5 receptor occupancy on peripheral blood bulk T cells (d), and monocytes (e). f, SARS-CoV-2 plasma viral load at days 0 and 7 post-leronlimab (n=7). Graph displays p-value calculated by Mann-Whitney test: *p ≤ 0.05, ** p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. (Source Patterson et al., Pre-Print)
A common theme in COVID-19 patients with severe disease is the rampant and out of control inflammatory signals. The vexing question is why some people respond like this and others do not. In a recent pre-peer manuscript, a study examined 10 terminally-ill, critical COVID-19 patients treated with to identify which cytokines and potential therapies could be applied to prevent this cytokine release syndrome (CRS). The authors report that these patients all had elevated IL-6 and CCL5 (RANTES), decreased CD8+ T cell levels, and SARS-CoV-2 plasma viremia. When patients were treated with a CCR5 blocking antibody (leronlimab), there was rapid reduction in plasma IL-6 and a significant decrease in SARS-CoV-2 plasma viremia. When they examined transcriptomic signatures in the myeloid compartment, using single-cell RNA-sequencing, they identified a reduction in IL-6 and Interferon-Related Genes. The authors conclude that “ These results demonstrate a novel approach to resolving unchecked inflammation………. via disruption of the CCL5-CCR5 axis…..”
Journal Article: Patterson et al., (Pre-Print). Disruption of the CCL5/RANTES-CCR5 Pathway Restores Immune Homeostasis and Reduces Plasma Viral Load in Critical COVID-19. MedRxiv
Article by Clive Gray










