Convalescent plasma therapy is the only antibody therapy available for COVID-19. Despite its use, the safety of this COVID-19 immunotherapy has not been assessed. Recent study by Joyner et al., “analyzed key safety metrics after transfusion of ABO-compatible human COVID-19 convalescent plasma in 5000 hospitalized adults with severe or life-threatening COVID-19, with 66% in the intensive care unit, as part of the US FDA expanded access program for COVID-19 convalescent plasma.”
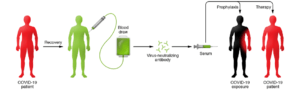
Figure 1: Schematic illustration for the proposed use of convalescent sera as therapy and prophylaxis in COVID-19. More information and various requirements for deployment and use of convalescent sera in COVID-19 can be found in Casadevall A and Pirofski (1).
Joyner et al., studied a cohort of 5000 participants, 81% with severe or life-threatening COVID-19% with a high risk of severe progression, who received convalescent plasma therapy. They reported a very low frequency (<1%) of severe adverse events with a 7-day mortality rate of 14.9%. Despite, the study not being designed to determine the efficacy of convalescent plasma therapy, as well as having a comparator group that didn’t receive therapy. Low SAE and mortality rate in range with rates observed by other (10-20% of hospitalised cases), suggests that convalescent is potentially safe, and does not contribute to increased pathology and potentially, mortality.
Journal Article: Joyner et al., 2020. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. JCI
Also read: Convalescent sera option for containing COVID-19 & Treating COVID-19 with immunoglobulins, should we be cautious?
Summary by Cheleka Mpande










