Humanity is currently confronting the COVID-19 pandemic caused by the novel coronavirus, SARS-CoV-2. There are no vaccines, monoclonal antibodies (Abs) or drugs available for treatment of COVID-19 as of yet. Global health research is therefore forced to focus on investigating various approaches that could be used to contain this deadly virus, and, indeed, many products are in rapid development, some of which, may be available in a short time.
According to Casadevall A and Pirofski, using convalescent sera as passive Ab therapy may be an option for the prevention and treatment of COVID-19 that could be available as soon as sufficient numbers of people recover and can donate immunoglobulin-containing serum.
Passive Ab therapy involves the administration of Abs against a given agent to a susceptible individual, to prevent or treat an infectious disease caused by that agent. Unlike active vaccination, which induces an immune response that takes time, this approach provides immediate immunity. Additionally, passive Ab therapy does not involve the time-consuming preparation of monoclonal Abs and other therapeutic products. Passive Ab therapy likely mediates protection via viral neutralisation or alternatively induce Ab-dependent cellular cytotoxicity and/or phagocytosis.
Convalescent sera is obtained from individuals who have recovered from a disease, and it is believed to be rich in Abs against the infectious agent that causes the disease. This has been used for treating various diseases, including in the 2009–2010 H1N1 influenza virus pandemic and the 2013 West African Ebola epidemic (2-4). Additionally, convalescent serum was safely used in previous coronavirus epidemics (SARS1 in 2002/2003 and MERS in 2012), where patients showed improved prognosis (5). COVID-19 convalescent sera could potentially be used to treat individuals with early symptoms and prevent disease in those exposed (prophylaxis), as shown in Figure 1. This is currently being tried in different regions including the US and China.
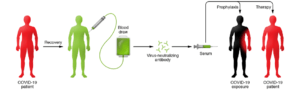
Figure 1: Schematic illustration for the proposed use of convalescent sera as therapy and prophylaxis in COVID-19. More information and various requirements for deployment and use of convalescent sera in COVID-19 can be found in Casadevall A and Pirofski (1).
Shen et al., (6) report a case study of 5 patients with severe COVID-19 who received convalescent Ab treatment. These patients received 2 consecutive doses (on the same day) of IgG and IgM Abs from 5 donors. All 5 donors were asymptomatic for at least 10 days with high SARS-CoV-2-specific Abs and nAbs titres confirmed using ELISA. Patients were all critical (4/5 on mechanical ventilation support) and on treatment with methylprednisolone and a combination of antivirals: lopinavir/ritonavir; interferon alfa-1b; favipiravir; arbidol and/or darunavir. Before Ab treatment patients had detectable Ab levels which increased upon Ab treatment. At the time of publication 3/5 patients had recovered and were discharged. Duan et al., (7) also tested the effectiveness of convalescent plasma therapy in severe COVID-19 patients. Similar to Shen et al., researchers tested this therapeutic strategy in severe COVID-19 patients (n=10) who were also on a combination of steroid, anti-viral, anti-fungal and/or anti-bacterial treatment. “One of the risks of plasma transfusion is the transmission of the potential pathogen. Duan et al., used Methylene blue photochemistry to inactivate the potential residual virus and to maintain the activity of neutralizing antibodies as much as possible.”
These studies highlight the potential of convalescent-Ab treatment, however small-size and co-administration of steroids and anti-bacterial/fungal/viral treatment prevent us from determining if patients improved specifically due to Ab-treatment. This emphasises the need for randomised clinical trials to investigate the efficacy of convalescent Ab treatment, before the treatment strategy can be endorsed.
References:
- Casadevall A and Pirofski LA. 2020. The convalescent sera option for containing COVID-19. JCI
- Luke TC et al., 2010. Hark back: Passive immunotherapy for influenza and other serious infections. Critical Care Medicine
- Hung IF, et al., 2009. Convalescent Plasma Treatment Reduced Mortality in Patients With Severe Pandemic Influenza A (H1N1) 2009 Virus Infection. Clin Infect Dis
- Kraft CS, et al., 2015. The Use of TKM-100802 and Convalescent Plasma in 2 patients with Ebola virus disease in the United States. Clin Infect Dis
- Wan Y et al., 2020. Molecular Mechanism for Antibody-Dependent Enhancement of coronavirus entry. J Virol
- Shen et al., 2020. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA.
- Duan et al., 2020. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. PNAS
Article by Eunice Kiambi & Cheleka AM Mpande