Introduction to Airway Goblet Cell Hyperplasia
Goblet cell hyperplasia (GCH) is a pathological condition characterised by an increased number of goblet cells in the airway epithelium, leading to excessive mucus production. This condition is a common feature of chronic respiratory diseases such as asthma and cystic fibrosis (CF), where it contributes significantly to morbidity by obstructing airways and impairing lung function. The excessive mucus produced by goblet cells can trap pathogens and particulate matter, leading to chronic infections and inflammation. Understanding the molecular and cellular mechanisms that drive GCH is crucial for developing targeted therapies aimed at reducing mucus hypersecretion and improving respiratory function in affected individuals.
Transcriptional Programs in Asthma and Cystic Fibrosis
Recent research has highlighted the role of distinct, disease-specific transcriptional programs in governing GCH in asthma and CF (Figure 1). In asthma, a chronic inflammatory disease characterised by airway hyperresponsiveness, Th2 cytokines such as IL-4 and IL-13 play a central role in driving GCH. These cytokines promote the expression of the transcription factor SAM-pointed domain-containing Ets-like factor (SPDEF), which is crucial for goblet cell differentiation. SPDEF activates a network of downstream genes involved in mucus production, thereby linking Th2-driven inflammation with GCH.
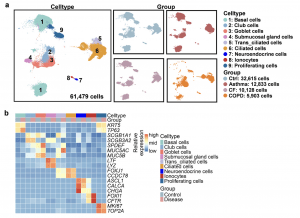
Figure 1: Single-cell RNA-sequencing analysis of airway epithelial cells in asthma, cystic fibrosis (CF), and chronic obstructive pulmonary disease (COPD). a Uniform manifold approximation and projection (UMAP) graphical representation of 61,479 epithelial cells from control and diseases. B Heatmap of canonical marker gene expression levels in nine airway epithelial cell types.
In contrast, CF, a genetic disorder caused by mutations in the CFTR gene, is associated with chronic bacterial infections and neutrophilic inflammation. The transcriptional programs governing GCH in CF are more complex and involve multiple signalling pathways. STAT6 and FOXA3 have been identified as key transcription factors in CF, regulating goblet cell differentiation in response to inflammatory signals. The chronic neutrophilic inflammation observed in CF patients drives the production of inflammatory cytokines such as IL-1β and TNF-α, which, in turn, activate STAT6 and other transcription factors involved in GCH.
The differential regulation of GCH in asthma and CF underscores the importance of disease-specific transcriptional programs in determining the extent and nature of mucus hypersecretion. These findings suggest that therapeutic strategies targeting these transcriptional programs could be tailored to the specific needs of patients with asthma or CF, potentially improving clinical outcomes.
Role of Inflammatory Mediators
Inflammation plays a critical role in the regulation of goblet cell differentiation and mucus production in both asthma and CF. However, the nature of the inflammatory response differs significantly between these diseases, leading to distinct patterns of GCH.
In asthma, the Th2-driven inflammation is characterised by the production of cytokines such as IL-4, IL-5, and IL-13. IL-13, in particular, is a key driver of GCH through its activation of the IL-13/STAT6/SPDEF signalling axis. This pathway promotes the differentiation of airway epithelial cells into goblet cells, leading to increased mucus production. The role of IL-13 in asthma has been well-established, and therapies targeting IL-13 or its receptor have shown promise in reducing GCH and mucus hypersecretion in asthma patients.
In contrast, CF is characterised by a more neutrophilic pattern of inflammation, driven by chronic bacterial infections. The persistent presence of pathogens such as Pseudomonas aeruginosa in the airways of CF patients leads to the production of pro-inflammatory cytokines like IL-1β and TNF-α. These cytokines activate multiple transcriptional pathways, including the STAT6 and NF-κB pathways, which promote goblet cell differentiation and mucus production. The resulting GCH contributes to the thick, viscous mucus that characterises CF, further exacerbating the cycle of infection and inflammation.
The distinct inflammatory mediators involved in GCH in asthma and CF highlight the importance of developing targeted therapies that address the specific inflammatory pathways driving mucus hypersecretion in each disease. For instance, while IL-13 inhibitors may be effective in reducing GCH in asthma, therapies targeting IL-1β or TNF-α may be more appropriate for CF patients.
Therapeutic Implications and Future Research
The identification of disease-specific transcriptional programs governing GCH has significant implications for the development of targeted therapies aimed at reducing mucus hypersecretion in asthma and CF. In asthma, therapies targeting the IL-13/STAT6/SPDEF pathway hold promise for reducing goblet cell differentiation and mucus production. Clinical trials of IL-13 inhibitors, such as dupilumab, have shown efficacy in reducing asthma symptoms and improving lung function by targeting this pathway.
In CF, therapeutic strategies need to address the chronic neutrophilic inflammation and the resulting GCH. Targeting the inflammatory mediators that drive GCH in CF, such as IL-1β and TNF-α, could help reduce mucus production and improve lung function. Additionally, therapies aimed at correcting the underlying CFTR defect, such as CFTR modulators, may also indirectly reduce GCH by improving ion transport and reducing airway surface liquid dehydration.
Future research should focus on further elucidating the transcriptional networks and signalling pathways involved in GCH in both asthma and CF. Understanding the crosstalk between different signalling pathways and identifying key regulatory nodes will be crucial for the development of more effective and personalised therapies. Additionally, exploring the role of epigenetic modifications and non-coding RNAs in the regulation of GCH may provide new insights into the molecular mechanisms underlying this condition.
Conclusion
Airway goblet cell hyperplasia is a common feature of chronic respiratory diseases such as asthma and cystic fibrosis, contributing to excessive mucus production and airway obstruction. Recent advances in our understanding of the transcriptional programs governing GCH have highlighted the role of disease-specific pathways in driving this condition. In asthma, the IL-13/STAT6/SPDEF axis is a key driver of GCH, while in CF, a more complex interplay of inflammatory mediators and transcription factors, including STAT6 and FOXA3, governs goblet cell differentiation.
The identification of these transcriptional programs has significant therapeutic implications, paving the way for the development of targeted therapies aimed at reducing mucus hypersecretion in asthma and CF. By addressing the specific inflammatory pathways driving GCH in each disease, these therapies have the potential to improve clinical outcomes and enhance the quality of life for patients with chronic respiratory diseases.
As research continues to unravel the molecular mechanisms underlying GCH, the hope is that more effective and personalised treatments will emerge, offering new hope to patients suffering from these debilitating conditions.
Journal article: Li., K., et al., 2024. Disease-specific transcriptional programs govern airway goblet cell metaplasia. Helyion.
Summary by Faith Oluwamakinde










