Researchers have found that a protein complex known to inhibit cancer cell growth also plays a critical role in slowing down tuberculosis (TB) infections. This protein complex, called GID/CTLH, impedes the growth of Mycobacterium tuberculosis (Mtb) within infected immune cells, enabling these cells to survive the infection (Figure 1). This breakthrough reveals a novel mechanism by which human cells defend against bacterial infections and offers potential for new therapeutic approaches in both cancer and TB treatment.
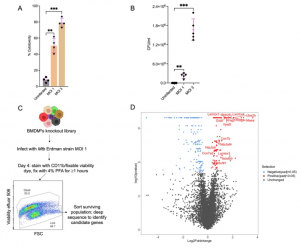
Figure 1: Genome-wide CRISPR screen to identify host determinants of tuberculosis restriction in macrophages. A Mycobacterium tuberculosis (Mtb) induced cytotoxicity in Hoxb8 bone marrow-derived macrophages (BMDMhs) at the indicated multiplicity of infections (MOI) 4 days post infection. Cytotoxicity was quantified by measuring the activity of released lactate dehydrogenase (LDH) in cell supernatants as compared to 100% LDH activity in detergent-lysed cells; n = 4 biological replicates. **P < 0.01; ***P < 0.001, one-way ANOVA alongside Dunnett’s multiple comparison test. Data are presented as mean values ± SD. B A further quantification of Mtb induced cytotoxicity in macrophages at indicated MOIs by plating colony forming units (CFUs) in cell supernatants from experimental conditions as in (A); n = 5 biological replicates. **P < 0.01; ***P < 0.001, one-way ANOVA alongside Dunnett’s multiple comparison test. Data are presented as mean values ± SD. C Schematic workflow for performing CRISPR screen to identify host determinants of Mtb restriction in macrophages. Part of this graphic was created in BioRender. Lee, B. (2024) BioRender.com/t32i914. D Volcano plot showing hits from the screen. For each gene (represented by dots), enrichment or depletion is shown in the x axis as log2 fold change while the y axis shows the corresponding −log10 p value. Significantly enriched and depleted hits are shown in red and blue colors, respectively, while unchanged hits are in gray. For significant hits, an adjusted p value of <0.05 was used as a cutoff. Screens were carried out in three independent replicates. Source data are provided as a Source Data file.
Previously, the GID complex was primarily associated with controlling glucose metabolism in yeast and targeting glucose degradation in human cancer cells, which rely heavily on glucose for growth. This study, however, is the first to implicate the GID complex in any infectious disease context.
The team discovered the complex’s role in immune defense through extensive screenings to identify new genetic targets that enable cells infected by Mtb to survive.
Using CRISPR/Cas9 technology, the team inactivated genes across the mouse genome within primary macrophages, which are key immune cells in fighting TB. The researchers then infected this population with Mtb, waiting until half of the macrophages had succumbed to the infection. They then examined the surviving cells, identifying genetic factors that contributed to their resistance.
This analysis revealed 259 genes that supported cell survival, including some known to bolster infection resistance and others not previously linked to this function. Among these were five genes encoding proteins that form the GID complex, confirming the GID pathway’s relevance in infection defense.
In addition to genetic screening, the team has identified several compounds that induce a similar metabolic shift to the GID-knockout, which could lead to new anti-TB drugs.
This discovery highlights the potential for cross-disciplinary advancements in drug development, underscoring the GID complex’s versatile role in both oncology and infectious disease therapy.
Journal article: Nelson V. Simwela, N.V., et al, 2024. Genome-wide screen of Mycobacterium tuberculosis-infected macrophages revealed GID/CTLH complex-mediated modulation of bacterial growth, Nature Communications.
Summary by Stefan Botha










