The mRNA-1273 vaccine is a novel lipid nanoparticle (LNP)-encapsulated mRNA-based vaccine that encodes for a full-length, prefusion stabilized full-length spike (S) protein of SARS-CoV-2 the virus that causes COVID-19. The vaccine mRNA-1273 candidate is co-developed by Moderna and Vaccine Research Centre based at the US-National Institutes of Health.
The Coronavirus Efficacy (COVE) phase 3 trial was launched in late July 2020 to assess the safety and efficacy of the mRNA-1273 vaccine in preventing SARS-CoV-2 infection. The trial was conducted at 99 centres in the United States. It was randomized, observer-blinded and placebo-controlled.
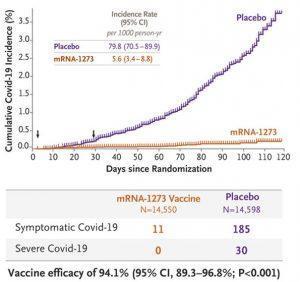
30,420 volunteers over 18 years old, were randomly assigned to receive either the vaccine or placebo in two intramuscular injections 28 days apart. The volunteers were followed for 2 months after the second dose to check for safety and laboratory-confirmed symptomatic COVID-19.
Efficacy was similar across key secondary analyses, including assessment 14 days after the first dose, analyses that included participants who had evidence of SARS-CoV-2 infection at baseline, and analyses in participants 65 years of age or older. Severe Covid-19 occurred in 30 participants, with one fatality; all 30 were in the placebo group. Moderate, transient reactogenicity after vaccination occurred more frequently in the mRNA-1273 group. Serious adverse events were rare, and the incidence was similar in the two groups.
The two doses of mRNA-1273 vaccine showed 94.1% efficacy at preventing COVID-19 illness including severe disease in persons 18 or older. Aside from transient local and systemic reactions, no safety concerns were identified.
The authors stated that there are still questions remaining and more research will be needed to understand the following:
- Safety and efficacy over a longer period of time, in a larger population, and in pregnant women and children
- Whether the vaccine protects against asymptomatic infection and transmission to unvaccinated persons
- How to care for those who miss the second vaccine dose
Journal Article: Baden et al, 2020. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. NEJM.
Also read our other articles about mRNA-1273:
- SARS-CoV-2 mRNA-1273 vaccine shows signs of potential efficacy
- Safety and immunogenicity of the SARS-CoV-2 mRNA-1273 vaccine candidate in older age
- NIH COVID-19 lecture on SARS-CoV-2 mRNA vaccine
- mRNA Vaccine against SARS-CoV-2 induces robust Ab responses
- Potential SARS-CoV-2 & COVID-19 Vaccines
Summary by Bon Holtak