Scientists have engineered a revolutionary approach to harnessing the body’s immune system against formidable diseases (Figure 1). By repurposing B cells, the immune cells responsible for antibody production, researchers have created a versatile platform capable of generating highly specific antibodies to combat a wide range of diseases.
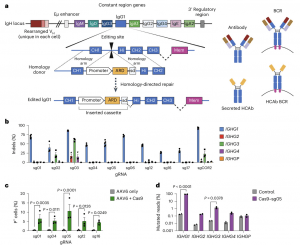
Figure 1: Genome editing at the constant region of the IgH locus. a, Antibody H chains are encoded by a rearranged variable domain (VH) spliced to a constant region that can be altered by class switch recombination. Alternate splicing within the constant region generates secreted antibody or membrane-anchored (BCR) isoforms. Genome editing to insert custom ARDs downstream of CH1 exons in the IgH constant regions can create HCAbs. The example shown is targeting human IgG1; the expanded view shows the constant region exons (bottom). Genome editing is directed by homology donors containing 500–750 bp homology arms flanking CRISPR–Cas9 target sites in the intron downstream of CH1. Homology-directed repair of the targeted DNA break inserts a cassette comprising a B cell-specific promoter, a custom ARD and a splice donor (sd) to direct splicing to downstream endogenous exons. Hi, hinge; Mem, membrane anchor. Created with Biorender.com. b, K562 cells were edited with Cas9 RNPs programmed by gRNAs targeting the IgG1 CH1 intron and indels measured at on-target (IGHG1) and off-target (IGHG2-P) genes by Sanger sequencing and inference of CRISPR edits (n = 3). c, K562 cells were edited by Cas9 RNPs plus AAV6 homology donors containing a GFP expression cassette, matched for each gRNA. GFP expression was measured after three weeks to dilute out episomal AAV genomes (n = 3). d, On- and off-target activity of sg05 was measured for the indicated IGHG genes in primary human B cells (n = 2) by targeted amplicon deep sequencing five days after editing with sg05 RNPs; the percentage of mutated reads was calculated as insertions, deletions and ≥2 bp changed. See also Extended Data Fig. 1d,e. b–d, Data are the mean ± s.e.m. c,d, Statistical comparisons were performed using a two-way analysis of variance (ANOVA) with Fisher’s least-significance difference test.
This breakthrough involves a precise genetic modification of B cells to transform them into potent, customizable disease fighters. Unlike traditional antibody therapies, this method allows for the creation of antibodies designed to outmanoeuvre even the most elusive pathogens, such as the rapidly mutating HIV virus.
Drawing inspiration from the success of CAR T cell therapy in cancer treatment, the researchers have adapted this concept for B cells. While CAR T cells excel at targeting rapidly dividing cancer cells, B cells offer a more sustained immune response due to their long-term presence in the body.
By employing CRISPR gene editing, scientists have introduced specific antibody blueprints directly into the B cell’s DNA, redirecting its antibody production capabilities. The resulting engineered B cells function as both vigilant sentinels and antibody factories, capable of persistent surveillance and enhanced antibody production when activated.
Proof-of-concept studies using human tissue have demonstrated the efficacy of this approach. This innovative technology holds immense promise for revolutionizing the treatment of a wide spectrum of diseases.
Journal article: Rogers, G. L., et al., 2024. Reprogramming human B cells with custom heavy-chain antibodies. Nature Biomedical Engineering.
Summary by Stefan Botha










