The Epstein-Barr virus (EBV), carried by 90% of adults, is notorious for causing infectious mononucleosis (“kissing disease”) and certain cancers. A new study sheds light on a potential strategy to combat EBV-linked malignancies by targeting the virus’s metabolic manipulation of infected cells (Figure 1).
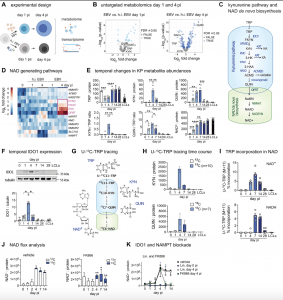
Figure 1: Metabolic profiling of EBV-infected B cells reveals transient up-regulation of NAD de novo biosynthesis in newly infected B cells. (A) Experimental design of metabolome and transcriptome analyses in naïve B cells infected with EBV or stimulated with heat-inactivated (h.i.) EBV, n = 6 biologically independent samples, paired analysis. (B) Comparison of metabolite abundances between EBV-infected and h.i. EBV-exposed naïve B cells, 1- and 4-days post infection (pi) (left and right panel, respectively). Colored dots indicate significantly changed metabolites [false discovery rate (FDR) ≤ 0.05]. Metabolites linked to NAD de novo biosynthesis are labeled: Nicotinamide adenine dinucleotide (NAD), tryptophan (TRP), l-kynurenine (KYN) and quinolinate (QUIN). (C) Scheme of the kynurenine pathway and interlinked NAD de novo biosynthetic pathway. (D) Hierarchical clustering of transcripts involved in NAD metabolism in EBV-infected naïve B cells and naïve B cells stimulated with h.i. EBV at days 0, 1, and 4 pi. Up-regulated transcripts involved in NAD de novo biosynthesis are highlighted and shown as log2 fold change to the average expression of the respective gene within the data set. (E) Metabolite abundance of TRP, KYN, QUIN, and ratios of KYN/TRP and QUIN/TRP, as well as NAD+ in bulk B cells, before and at 5 time points after infection, as indicated, and compared to lymphoblastoid cell lines (LCLs). Data are represented as mean values of indicated individual data points (n = 6 independent experiments. (F) Representative immunoblot (upper panel) and pooled data from n = 4 independent experiments (lower panel) of Indoleamine 2,3-Dioxygenase 1 (IDO1) expression in bulk B cells, before and at 5 time points after infection, as indicated, and compared to LCLs. Data are shown as median values of indicated individual data points. (G) Schematic of tracer incorporation into the kynurenine pathway and interlinked NAD de novo biosynthetic pathway using uniformly 13C-labeled tryptophan (U-13C-TRP). (H) Fraction of 13C-labeled KYN (m+10) (upper panel) and QUIN (m+7) (lower panel) at indicated time points after infection of bulk B cells, and in LCLs, normalized to total protein. Data are shown as median values and range, n = 4 independent experiments. (I) U-13C-TRP incorporation into total cellular NAD+ (upper panel) and NADH (lower panel). Data are shown as median values of indicated individual data points (n = 4 independent experiments). (J) U-13C-TRP incorporation into total cellular NAD+ after blockade of NAD recycling with FK866 (added at day 0 pi) (right panel) and with vehicle control (left panel) displayed as medians. (K) Total NAD+ levels as assessed by mass spectrometry in EBV-infected bulk B cells before and at 5 time points after infection as indicated, treated with vehicle, Lin. added at day 0 pi, Lin. added at day 4 pi, and FK866 added at day 4 pi. Data are shown as median of 4 independent experiments, comparing vehicle and Lin. day 4 pi (black stars), vehicle and Lin. day 0 pi (blue stars), and vehicle and FK866 day 4 pi. Experiments (A), (B), and (D) were performed using naïve B cells, experiments (E) to (K) using bulk B cells. Data in (E) and (F) were compared using a repeated measures ANOVA test followed by multiple comparisons test, data in (J) and (K) were compared using a one-way ANOVA followed by multiple comparisons test. P-values are indicated as: * P ≤ 0.05, ** P ≤ 0.001, *** P ≤ 0.0001, **** P ≤ 0.0001.
Researchers have unraveled how EBV reprograms infected B cells, a process crucial for chronic infection and cancer development. They discovered that the virus jumpstarts the production of an enzyme called IDO1 within infected cells. IDO1 activity fuels the cell’s power plants (mitochondria), generating extra energy needed for the virus’s manipulations and the rapid multiplication of reprogrammed B cells.
The study focused on a group of organ transplant patients who developed EBV-associated blood cancer after immunosuppressive therapy (needed to prevent organ rejection). Interestingly, the researchers found that EBV increased IDO1 levels months before cancer diagnosis. This discovery holds promise for developing biomarkers for early cancer detection.
The study’s key finding is the role of the IDO1 pathway in EBV-mediated cancer development. This suggests that inhibiting this pathway could be a potential therapeutic strategy. By curbing the extra energy production fueled by IDO1, researchers hope to hinder the virus’s ability to reprogram B cells and reduce the risk of EBV-associated cancers.
This research paves the way for further investigation into targeting the EBV-IDO1 pathway as a potential approach to preventing EBV-linked cancers, offering new hope for individuals at risk.
Journal article: Müller-Durovic, B, et al., 2024. A metabolic dependency of EBV can be targeted to hinder B cell transformation. Science.
Summary by Stefan Botha










