T-cell immunity plays a crucial role in controlling COVID-19, especially in individuals who cannot generate antibody responses. Researchers have recently developed a peptide based T cell vaccine candidate CoVac-1. CoVac-1 is an activator of T-cells, composed of specific immunological epitopes from the SARS-CoV-2 virus. This candidate vaccine has been shown to be safe and effective in promoting a targeted T-cell response against SARS-CoV-2. In this report, the author present findings from a Phase I/II clinical trial (NCT04954469) involving 54 patients with either congenital or acquired B-cell deficiency. These patients were administered a single subcutaneous dose of CoVac-1 (Figure 1).
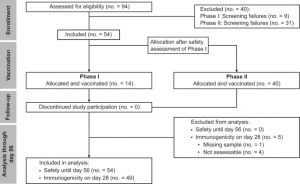
Figure 1: Consort flow diagram of the trial: 40 patients did not meet the inclusion criteria at screening and accordingly were not enrolled in the trial. All 54 enrolled patients received one dose of CoVac-1. Safety oversight to proceed to Phase II was performed by an independent data and safety monitoring board and approved by the competent authority (Paul Ehrlich Institute) and the local Ethics Committee after an interim safety and immunogenicity analysis of study patients included in Phase I, evaluated on day 28 after CoVac-1 application. Five patients were not assessable for the primary endpoint analysis of immunogenicity. All enrolled patients were assessable for safety. no., number.
The primary goal was to assess the CoVac-1-induced T-cell responses, while safety was a secondary consideration. Notably, no severe or critical adverse events linked to CoVac-1 were observed. While 94% of participants experienced localized granuloma formation, this was an anticipated response. Systemic reactions were either mild or absent. Impressively, 86% of patients displayed SARS-CoV-2- specific T-cell responses initiated by CoVac-1. These responses targeted various CoVac-1 peptides, demonstrating resilience against current Omicron variants. The responses were facilitated by multifunctional T-helper 1 CD4+ T cells. Remarkably, the T-cell responses triggered by CoVac-1 exceeded those provoked by spike protein-focused mRNA-based vaccinations in B-cell deficient patients and COVID-19 convalescents with or without antibody production. Overall, the findings reveal that CoVac-1 effectively generates robust and comprehensive T-cell responses in patients lacking B-cells or antibodies, all while maintaining a positive safety profile.
The research successfully demonstrates that CoVac-1, a peptide-based T-cell activator, elicits potent and broad SARS-CoV-2-specific T-cell responses in B-cell deficient patients, outperforming spike protein-based vaccinations and indicating promise for further development. These results underscore the need for further evaluation in Phase III trials to gauge safety and efficacy. The clinical trial is registered under NCT04954469 on ClinicalTrials.gov.
Journal article: Heitmann, J.S., et al., 2023. Phase I/II trial of a peptide-based COVID-19 T-cell activator in patients with B-cell deficiency. Nature Commun.
Summary by Dr. Gupreet Kaur










