Mutations in the p53 tumor suppressor gene not only drive the growth of cancer cells but also impact the microenvironment surrounding tumours. Recent research reveals that breast cancer cells with p53 mutations can alter fat cells, leading to the development of an inflammatory environment that supports cancer growth by suppressing the immune response (Figure 1).
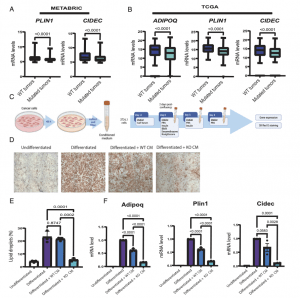
Figure 1: p53 loss in murine breast cancer cells inhibits adipocytic differentiation. (A) Relative abundance of PLIN1 and CIDEC mRNA in p53wt breast tumors (n = 1,236) versus p53 mutated tumors (n = 653) in the METABRIC dataset. Two- sample t test. Note: The METABRIC microarrays do not include ADIPOQ probes. (B) Relative abundance of ADIPOQ, PLIN1, and CIDEC mRNA in p53wt breast tumors (n = 638) versus p53 mutated tumors (n = 327) in TCGA dataset (BRCA cohort). Two-sample t test. (C) Protocol for 3T3- L1 differentiation in the presence of conditioned medium (CM) from parental or Trp53 knockout mouse breast cancer cells (WEA or WEP cell lines). Figure created with https://www.BioRender.com. (D) Oil- Red- O staining of undifferentiated 3T3- L1 preadipocytes, 3T3- L1 cells induced to differentiate in the presence of regular differentiation medium (Differentiated) or differentiation medium supplemented (1:1 ratio) with CM from WT or p53KO WEA cells. (E) Quantification of lipid droplets in the cell cultures in (D). Quantification was done with ImageJ Macro script. Mean + SEM from three biological repeats (one- way ANOVA and Tukey’s post hoc test). (F) qRT- PCR analysis of mature adipocyte marker mRNAs in the cells in (D). Values were first normalized to Nono mRNA in the same samples and are shown relative to 3T3- L1 preadipocytes undergoing regular differentiation. Mean + SEM from three biological repeats (one- way ANOVA and Tukey’s post hoc test).
P53 mutations are prevalent in human tumours, occurring in approximately 30% of breast cancer cases. These mutations hinder the ability of p53 to prevent the development and progression of cancer. While much is known about the direct effects of these mutations on cancer cells, their influence on the tumour microenvironment is an area of ongoing investigation.
The focus of this study was on understanding how p53 mutations in breast cancer cells affect adipocytes, or fat cells. The findings suggest that these mutations increase the aggressiveness and resistance to therapy of neighboring breast cancer cells.
An interesting observation was that specific p53 mutations in breast cancer cells could reprogram adjacent precursor fat cells, inducing a more pronounced pro-inflammatory state compared to breast cancer cells without functional p53.
The study emphasizes that p53 defects within breast cancer cells play a central role in reshaping fat cells, creating an environment favourable for tumour progression. Gaining insights into the interaction between p53-mutated cancer cells and adipocytes could potentially inform strategies to impede the advancement of breast cancer.
Journal article: Hassin, O., et al., 2023. p53 deficient breast cancer cells reprogram preadipocytes toward tumor-protective immunomodulatory cells. Proceedings of the National Academy of Sciences.
Summary by Stefan Botha










