Fever, a common physiological response to infection or inflammation, is often viewed as a beneficial mechanism to combat pathogens. However, researchers have revealed a more complex interplay between fever and immune cell function (Figure 1).
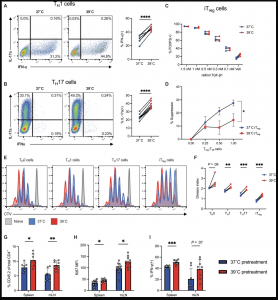
Figure 1: CD4 T cell function is broadly altered at febrile temperatures. (A) IFN-γ expression by TH1 cells after 3 to 4 days of culture at 37° or 39°C (n = 8). (B) IL-17A expression by TH17 cells after 3 to 4 days of culture at 37° or 39°C (n = 10). (C) FOXP3 expression of iTregs cultured in an hTGF-β dilution series (n = 4). (D) Suppression assay of activated CD8 T cells performed at 37°C, cocultured with CD4+ iTregs previously cultured at 37° or 39°C (n = 4). (E) CTV staining of CD4 T cell subsets cultured for 3 days at 37° (blue) or 39°C (red). Naïve (gray) CD4 T cells served as a nonproliferating control. (F) Division index calculated using CTV data in (E) (n = 4). (G to I) CD45.2+ OT-II CD4 T cells were activated, cultured in vitro for 3 days at 37° or 39°C, and then transferred intravenously into CD45.1 hosts. Recipient animals were then immunized intraperitoneally with ovalbumin in CFA, and spleens and mLNs were analyzed after an additional 4 days (n = 12 for 37°C and n = 10 for 39°C pretreatment). (G) Percentage of CD45.2+ cells measured in spleens and mLNs. (H) Ki67 mean fluorescence intensity (MFI) of CD45.2-expressing cells in the spleens and mLNs of CD45.1+-recipient mice. (I) Percentage of IFN-γ+ cells of total CD45.2+ adoptively transferred cells in the spleens and mLNs. All biological replicates (n) are shown with SDs and means and are representative of at least two independent experiments, unless otherwise stated. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Paired t test [(A), (B), and (F)] and unpaired t test [(D) and (G) to (I)].
Historically, the impact of fever on cellular processes has been relatively understudied. Most research in this area has focused on agriculture, investigating how extreme temperatures affect crops and livestock. In the context of human health, studying the effects of fever in animal models can be challenging due to the stress it induces. Additionally, laboratory cells are typically cultured at a constant temperature of 37 degrees Celsius, which is the normal human body temperature.
They explored the effects of fever due to his father’s experience with an autoimmune disease that caused prolonged fever. They cultured immune system T cells at a higher temperature of 39 degrees Celsius and observed that this heat increased the activity of helper T cells, which are essential for fighting infections, while reducing the suppressive activity of regulatory T cells.
However, the researchers made a surprising discovery: a specific subset of helper T cells, known as Th1 cells, experienced mitochondrial stress, DNA damage, and even cell death. This was perplexing because Th1 cells are crucial for combating viral infections, which often involve fever.
Further investigation revealed that only a portion of Th1 cells succumb to these detrimental effects. The surviving cells undergo adaptations to their mitochondria, becoming more resistant to stress and exhibiting increased proliferation and cytokine production.
They identified the molecular mechanisms underlying this cellular response to fever temperatures. He found that heat rapidly impairs a mitochondrial protein complex called ETC1, which is responsible for generating energy. This impairment triggers signaling pathways that lead to DNA damage and activation of the tumor suppressor protein p53, which either repairs DNA or induces cell death to maintain genome integrity. Th1 cells were found to be particularly sensitive to ETC1 impairment.
Interestingly, similar changes in Th1 cells were observed in patients with Crohn’s disease and rheumatoid arthritis, supporting the molecular signaling pathway identified in the study.
The researchers believe that this cellular response to heat is a fundamental mechanism by which cells sense and respond to stress. Temperature variations across tissues and over time can influence cellular metabolism and function, potentially impacting a wide range of biological processes.
The findings of this study suggest that prolonged exposure to elevated temperatures, as seen in chronic inflammation, could contribute to cancer development by inducing DNA damage and impairing cellular repair mechanisms. This research provides valuable insights into the complex relationship between fever, immune cell function, and human health.
Journal article: Heintzman, D.R., 2024. Subset-specific mitochondrial stress and DNA damage shape T cell responses to fever and inflammation. Science Immunology.
Summary by Stefan Botha










