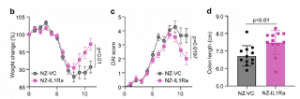
Effect of oral administration of NZ-IL1Ra on DSS-induced colitis in model. Change in body weight and DAI score (b and c respectively), of mice with DSS-induced colitis. Data are the mean ± SE (n = 18). The p values of day 11 results are shown. Colon length on day 11 (d). Data are the mean ± SE (n = 12), and each dot in the plot represents one mouse. (Source: Namai, F. et al., 2020)
Inflammatory bowel disease (IBD), which includes Crohn’s disease (CD) and ulcerative colitis (UC), is a complex inflammatory disorder of the gastrointestinal tract. The incidence of IBD is on the rise worldwide and possible triggers of the disease include an inflammatory response caused by an infection with a specific pathogen or having a defective mucosal barrier. The precise mechanism for the development and progression of IBD remains unclear, however, there has been increasing evidence suggesting that the imbalance between pro- and anti-inflammatory cytokines leads to disease progression and tissue destruction.
Although there are several biologics available for the treatment of IBD, their therapeutic potential is affected by a decrease in response and severe side effects induced by the parenteral high dose administration. Improving the efficacy of therapeutics and enabling localised treatment requires the use of a drug delivery system that will locally deliver and release molecules at the intestinal mucosa and function as a protection barrier from the stomach’s low pH environment. A recent study by Namai, F. et al., has developed a novel IBD therapeutic using a microbe as a protein delivery system.
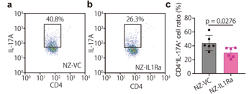
Anti-inflammatory effect of NZ-IL1Ra on cytokine expression. Mesenteric lymph nodes excised on day 11 were stained with an Alexa Fluor488 anti-mouse CD4 antibody and a PerCP/Cy5.5 anti-mouse IL-17A antibody. Representative flow cytometry dot plots of CD4+ IL-17A+ cells in NZ-VC (a) and NZ-IL1Ra groups (b) are shown. Graph showing the average percentage of CD4+ IL-17A+ cells (c). Data are the mean ± SE (n = 6), and each dot in the plot represents one mouse. (Source: Namai, F. et al., 2020)
In their study the authors, constructed a genetically modified lactic acid bacteria (gmLAB) (designated NZ-IL1Ra) that secretes high levels of recombinant mouse Interleukin 1 receptor antagonist (rmIL-1Ra) which inhibits the immune cells that attack the intestinal lining in IBD patients. They investigated the efficacy of the rmIL-1Ra using a DSS-induced acute colitis mouse model. Mice with DSS-induced colitis were orally administered NZ-IL1Ra or the control (gmLAB lacking the mIL-1Ra gene and designated NZ-VC). The authors found that oral administration of NZ-IL1Ra suppressed body weight reduction and the disease activity index (DAI) score in mice with acute colitis significantly decreased. Likewise, the oral administration of NZ-IL1Ra also decreased the number of CD4+ IL-17A+ cells in the mesenteric lymph nodes and significantly improved the colitis-associated shortening of the colon.
In summary, the authors showed that the genetically modified Lactococcus lactis can facilitate the efficient delivery of IL-1Ra resulting in reduced inflammation and associated symptoms in a mouse model of ulcerative colitis.
Journal Article: Namai, F. et al. (2020) Microbial therapeutics for acute colitis based on genetically modified Lactococcus lactis hypersecreting IL-1Ra in mice. Exp Mol Med
Article by Lorraine Pfavayi
Other references
- Cao, J., et al. (2019). “Alginate/chitosan microcapsules for in-situ delivery of the protein, interleukin-1 receptor antagonist (IL-1Ra), for the treatment of dextran sulfate sodium (DSS)-induced colitis in a mouse model.” European Journal of Pharmaceutics and Biopharmaceutics 137: 112-121.
- Cabral, V. P. et al. (2016). “Severe infection in patients with rheumatoid arthritis taking anakinra, rituximab, or abatacept: a systematic review of observational studies.” Revista brasileira de reumatologia 56(6): 543-550.
- Yu, Y. R. and J. R. Rodriguez (2017). “Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes.” Seminars in Pediatric Surgery 26(6): 349-355.










