Chronic inflammation may increase the risk of developing many neoplasia such as breast cancer, by inducing reactive oxygen species that induce DNA damage and neo angiogenesis. Previous studies have highlighted a potential link between key molecular factors in the inflammatory pathway and the risk for breast cancer. In particular, TLR4 expression and mutations have been associated with an increased risk for breast cancer. However these results still mitigated and show a variability from one population to another, thus highlighting implication of environmental bias (1). Hence, it is primordial to elaborate study design that decrease the impact of confounding factors to better address the question of the relation between the expression levels of the molecular factor of interest and the risk for neoplasia.
A study by Su et al has demonstrated that using a Mendelian randomization approach reduce environmental bias on the causal relationship between genetically elevated CRP levels and postmenopausal breast cancer risk (2).
The originality in their approach come from: instead of trying to link CRP levels with breast neoplasia risk, they first define genetic variants of CRP gene that are associated with chronic low grade inflammation (CRP equal to or greater than 3mg/L). These variants were grouped and considered as the mutated or alternative allele. The other alleles associated with low CRP level (under 3mg/L) were considered as the reference allele. And then, through the Medelian Randomization (MR), they investigate the influence of these alleles on the risk for breast neoplasia. The MR reduce bias since usually the gene transmitted through the gametes aren’t under influence of the tumor growth, the environmental and behavioural factors. Indeed, with this approach the statistical power of the randomized clinical trial is reachable.
The data were extracted from five GWAS (genome wide association studies). Of a total of 16,088 women who reported their race or ethnicity as non-Hispanic white, they applied exclusion criteria (history of diabetes, genomic data quality control, less than 1 year follow-up of cancer outcomes, and diagnosis of any cancer type at screening), resulting in a total of 10,179 women. They had been followed during a mean of 16 years and 537 (5% of the eligible 10,179 women) developed primary invasive breast cancer. The 61 alleles tested in this study were independent (linkage disequilibrium < 0.3). Stratified analysis were defined by potential effect modifiers, including lifestyle factors and breast cancer molecular subtypes, and conducted by taking into account the obesity related factors and sex hormone-lifestyle.
This study seems to be the first to report the causal effect of genetically elevated CRP levels on increased breast cancer risk in an MR framework (Table I). When the patients were oral contraceptive estrogen and or progestin users for less than 5 years, the risk for breast cancer was decreased by low grade chronic inflammation status. Estrogen-only users for ≥5 years had more profound CRP-decreased breast cancer risk in dose–response fashion. Also, genetically predicted CRP was strongly associated with increased risk for hormone-receptor positive or human epidermal growth factor receptor-2 negative breast cancer.
In conclusion, Mendelian Randomization (MR) approach could be used to investigate the risk of cancer from immune system components that are under influence of environmental, behavioural and tumoral factors with reliability of randomized clinical trials. On the other hand, this study raise a question about the people from certain low and middle income countries that are chronically under exposure to viral, bacterial and parasitic pathogens, are they at higher risk for hormone-receptor positive or human epidermal growth factor receptor-2 negative breast cancer?
Table 1: Mendelian randomization analysis: the effect of genetically predicted chronic inflammation status on breast cancer risk by exogenous estrogen plus progestin use.
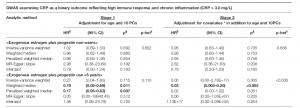
CI, confidence interval; CRP, C-reactive protein; GWAS, genome-wide association study; HR, hazard ratio; MR, Mendelian randomization; PCs, principal components; SFA, saturated fatty acids; SNP, single-nucleotide polymorphism. Numbers in bold face are statistically significant. *Covariates adjusted in the analyses for the association between genome-wide SNPs and breast cancer risk include education; annual family income; family history of breast cancer; body mass index; waist-to-hip ratio; physical activity; depressive symptoms; number of cigarettes per day; dietary alcohol in g/day; % calories from SFA/day; age at menopause; duration of oral contraceptive use; and duration of exogenous estrogen-only use. §p values were adjusted to correct for multiple testing via the Benjamini–Hochberg approach. ¶The Mendelian randomization estimate (except weighted/penalized weighted medians) was adjusted for a correlation between CRP phenotype and breast cancer risk within the same population. †Heterogeneity in estimates among genome-wide SNPs was evaluated with Cochran’s Q test with fixed effects.
References
(1) Pandey, N., Chauhan, A., & Jain, N. (2018). TLR4 polymorphisms and expression in solid cancers. Molecular diagnosis & therapy, 22(6), 683-702.
(2) Jung, S. Y., Papp, J. C., Sobel, E. M., Pellegrini, M., Yu, H., & Zhang, Z. F. (2020). Genetically predicted C-reactive protein associated with postmenopausal breast cancer risk: interrelation with estrogen and cancer molecular subtypes using Mendelian randomization. Frontiers in oncology, 10, 3354.
Journal article: Jung, et al., 2020. Genetically predicted C-reactive protein associated with postmenopausal breast cancer risk: interrelation with estrogen and cancer molecular subtypes using Mendelian randomization. Frontiers in oncology.
Summary by Doudou G. M. Niang










