The trillions of microbes residing in our gut, known as the gut microbiota, play a far more significant role than just aiding digestion. They produce vital enzymes, metabolites, and other molecules that influence our immune and endocrine systems, impacting our overall health. A recent study sheds light on a surprising new connection: a father’s gut health may influence the health of his offspring (Figure 1).
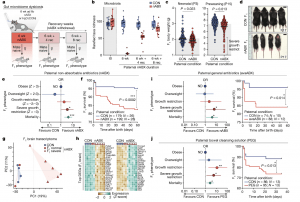
Figure 1: Paternal gut dysbiosis probabilistically triggers major F1 phenotypes. a, Schematic showing the strategy for induced paternal dysbiosis and recovery using nABX. b, Quantification of microbial taxa richness in males after 6 weeks of nABX treatment and during the recovery (rec) time course, by 16S rRNA sequencing (CON t0 = 18, 6 wk = 18, 6 wk + 4 rec = 14, 6 wk + 8 rec = 11; nABX t0 = 19, 6 wk = 12, 6 wk + 4 rec = 13, 6 wk + 8 rec = 12 individuals per timepoint). Bar represents median, whiskers 1.5× interquartile range. c, Body weight of F1 offspring at postnatal days P3 and P15 according to paternal nABX treatment. P value by two-tailed nested (hierarchical) t-test (CON n = 172, nested into N = 26 fathers; nABX n = 181 nested into N = 28 fathers). Bar indicates mean. d, Representative images of SGR phenotype in F1 offspring from dysbiotic fathers (nABX-treated). e, Forest plot showing the log OR of risk for abnormal body-weight classes in offspring that survive to P15. Null effect is represented by a vertical line for which OR value is 1. Whiskers indicate 95% CI, P value by two-sided Chi-square test (mortality P = 0.0001; SGR P = 0.044). f, Kaplan–Meier plot showing postnatal survival of F1 progeny depending on paternal nABX treatment regime (CON n = 179; nABX n = 199). P value by Mantel–Cox (log-rank) test. g, PCA of transcriptomes from F1 brains derived from control or nABX sires. The nABX offspring are stratified by normal or SGR phenotype. SGR not observed in control offspring. h, Heatmap showing expression of top upregulated and downregulated genes in F1 SGR brains, from independent litters sired by nABX-treated fathers. i, Left, OR of F1 susceptibility for abnormal body weight and mortality when sired by males with dysbiosis induced by 6 weeks of treatment with general antibiotics (avaABX). Whiskers indicate 95% CI, P value by two-sided Chi-square test (mortality P = 0.014; SGR P = 0.038). Right, Kaplan–Meier plot showing postnatal survival of F1 progeny from avaABX-treated males. j, Left, OR of F1 risk for abnormal body weight when sired by males with dysbiosis induced by 6 weeks of bowel cleansing with PEG laxative. Whiskers indicate 95% CI, P value by two-sided Chi-square test (mortality P = 0.013; SGR P = 0.014). Right, Kaplan–Meier plot showing survival of F1 progeny from PEG-treated males. n, offspring; N, litters; ND, not detected.
While the importance of a balanced gut microbiota for the mother’s health during pregnancy is recognized, little was previously known about the impact of a father’s gut microbes on his offspring. This study in mice revealed that disrupting the gut microbiota in male mice through targeted antibiotics led to concerning outcomes for their offspring.
Researchers induced a state called dysbiosis in male mice by treating them with antibiotics that don’t enter the bloodstream. Dysbiosis refers to an imbalanced gut microbial ecosystem. The study revealed that this disruption in the fathers’ gut microbiota negatively affected their testicular physiology, metabolite composition, and hormonal signaling. Leptin, a key hormone, showed altered levels in both the blood and testes of these mice. These findings suggest a “gut-germline axis” in mammals, a communication pathway linking the gut, its microbes, and the reproductive system.
The study’s most striking finding was the impact of paternal dysbiosis on offspring health. When dysbiotic males were mated with untreated females, their pups exhibited significantly lower birth weights and a higher rate of death shortly after birth. These negative effects were observed regardless of the specific antibiotic combination used or whether laxatives were employed to induce gut microbiota disruption.
Encouragingly, the study also demonstrated the reversibility of these effects. Once the antibiotics were stopped, the fathers’ gut microbiota recovered. Subsequently, when these males with restored gut health mated with untreated females, their offspring displayed normal birth weights and healthy development.
The study delved deeper, revealing more concerning implications. Pregnancies involving dysbiotic fathers showed a higher incidence of placental defects, including compromised blood vessel formation and reduced growth. These defects mirrored hallmarks of pre-eclampsia, a human pregnancy complication leading to impaired fetal growth and increased risk of later-life diseases in offspring.
This study establishes a communication channel between the gut microbiota and the male reproductive system in mice. Environmental factors disrupting these signals in fathers can negatively impact offspring health through altered placental development. These findings suggest that a father’s environment just before conception can influence offspring traits independent of genetics. Further research is needed to determine if similar mechanisms exist in humans and explore potential interventions to optimize paternal gut health for the benefit of future generations.
Journal article: Argaw-Denboba, A., et al. 2024. Paternal microbiome perturbations impact offspring fitness. Nature.
Summary by Stefan Botha










