In an interesting discovery that has broad implications ranging from improving vaccine efficacy to the potential treatment of digestive diseases, researchers from the University of Texas Southwestern Medical Center have discovered how vitamin A enters immune cells in the intestines. This discovery may allow for the manipulation of vitamin A delivery into the immune system for disease treatment and prophylaxis. (Read more: The Immunoregulatory Role of Vitamin A and Retinoic Acid in the Mucosal Immune System and Mucosal Immunity & Immunopathology).
Vitamin A, a lipid-soluble nutrient, is absorbed by intestinal epithelial cells and converted to retinol and subsequently converted to retinoic acid before it may be utilised by the tissues of the body. Retinol is essential for intestinal adaptive immunity because it directs the development of intestinal B and T cells and promotes their recruitment to the intestine. Adaptive immune system forms a subset of the broader immune system, reacting to pathogens based on immunological memory following previous exposure to pathogenic organisms and/or vaccines. A deficiency in vitamin A has been associated with an increased susceptibility to infectious diseases, specifically those of the intestine. Myeloid cells are central to the development of vitamin A–dependent intestinal immunity. Myeloid cells include dendritic cells (DCs) and macrophages, which circulate in the intestine and present antigens to naïve B and T cells to promote their differentiation into functional immune cells.
Bang, et al., sought to answer the question of how myeloid cells acquire retinol in order for the conversion of retinol to retinoic acid for use in the body. For this they focused on a family of retinol-binding proteins/receptors i.e. serum amyloid A proteins (SAA) which are produced during infection. In this present study they identified low-density lipoprotein (LDL) receptor–related protein 1 (LRP1) as an SAA protein receptor that endocytoses SAA-retinol complexes and promotes retinol acquisition by retinoic acid-producing intestinal myeloid cells.
Bang, et al., reported that LRP1 was expressed by intestinal myeloid cells, where it acts as a means of transport for retinol. Using knockout mice specific for this receptor, myeloid cells could not take up retinol leading to the eradication of the adaptive immune system in their gut. In addition to this, there was a significant reduction in the population of T and B cells as well as the molecule immunoglobulin A, all critical components of adaptive immunity.
Following this initial discovery, the researchers went on to compare the response to Salmonella Typhimurium infection between mice with LRP1 and knockout mice, lacking LRP1. In short, the knockout mice were more susceptible to infection. This highlighting that SAAs and LRP1 promote the development of vitamin A—dependent adaptive immunity in the intestine and enhance resistance to enteric infection after immunization (Figure 1).
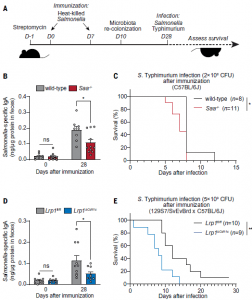
Figure 1: SAA and LRP1 promote immunity to enteric bacterial infection. (A) Wild-type and Saa−/− mice [(B) and (C)] and Lrp1fl/fl and Lrp1DCd11c (knockout) mice [(D) and (E)] were immunized with 1010 CFU of heat-killed S. Typhimurium twice through oral gavage. Four weeks after the first immunization, the mice were orally infected with 2 × 108 CFU (C) and 5 × 109 CFU (E) of log-phase S. Typhimurium (the infection dose differs because resistance to infection differs according to mouse background). (B and D) S. Typhimurium–specific fecal IgA was measured by ELISA. Means ± SEMs are plotted. *P < 0.05; Student’s t test. (C and E) Survival rates were monitored after infection. Data represent results from five cohoused cages of wild-type and Saa−/− littermates [(B) and (D)] and five cohoused cages of Lrp1fl/fl and Lrp1DCd11c littermates [(D) and (E)]. *P <0.05; **P < 0.01; ns, not significant; log-rank test (Bang, et al., 2021).
Looking at the implications of their work it has been brilliantly summarised in their own words:
“…by illuminating how vitamin A is mobilized to the intestinal immune system, our results may offer additional approaches for enhancing resistance to infection and for increasing the efficacy of mucosal vaccines, which depend on adequate dietary vitamin A (45). Our findings also suggest the possibility of selectively regulating the availability of retinol to the immune system during inflammatory disease, perhaps by deploying small molecule inhibitors of the SAA-retinol or SAA-LRP1 binding interactions.”
A summary of these findings can be seen in Figure 2.
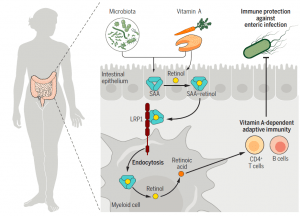
Figure 2: SAA proteins deliver retinol to intestinal myeloid cells through LRP1 to promote adaptive immunity. SAAs are retinol-binding proteins induced in intestinal epithelial cells by the microbiota and vitamin A. SAAs deliver retinol to retinoic acid-producing myeloid cells by binding to the endocytic receptor LRP1. LRP1 and SAAs promote development of vitamin A–dependent adaptive immunity in the intestine and enhance resistance to enteric infection (Bang, et al., 2021).
Journal Article: Bang, et al., 2021. Serum amyloid A delivers retinol to intestinal myeloid cells to promote adaptive immunity. Science Advances.
Summary by Stefan Botha










