Hepatitis E is a liver infection caused by the Hepatitis E virus (HEV). While most people recover fully, it can be chronic in immunocompromised individuals, like those with organ transplants or certain cancers. There’s also no specific and consistently effective treatment. A new study sheds light on how HEV evades the immune system and potentially transitions from acute to chronic infection (Figure 1).
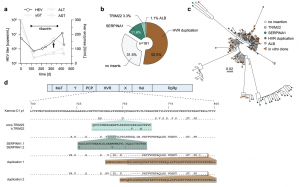
Figure 1: HVR insertions in a HEV patient identified by clonal sequencing. a The treatment course of a chronically HEV-infected solid organ recipient is shown. The solid black line indicates the viral titer as copies/mL, while the grey lines visualize the liver enzyme levels IU/mL. The treatment period with ribavirin is indicated by the bar above. The arrow highlights the time point used for clonal sequencing. b The distribution of HVR rearrangements identified in HEV-positive colonies is shown. Depicted is the origin of inserted sequences (genes TRIM22, SERPINA1, and HEV HVR duplication) and their frequency as a percentage. Source data are provided in the Source Data file. c Maximum likelihood tree of amplicon-based clonal sequencing derived variants. Insertion-carrying clones are depicted in cyan (TRIM22), green (SERPINA1), and brown (duplication). Sequences of engineered in vitro clones representing respective clusters are depicted as orange triangles. The scale bar indicates the average number of substitutions per site. d The insertion site as well as their similarity (dots) to the strain Kernow-C1-p1 are shown. Mismatches to the reference are indicated by the used amino acid as a single letter code. The inserted amino acid sequence is indicated below. The name of the constructs is indicated in front of each row. Black boxes refer to duplicated sequence snippets.
The team closely monitored a patient with chronic HEV for over a year. They analyzed the genetic makeup (RNA) of the virus over time. They discovered that HEV incorporated snippets of the patient’s own RNA into its genome. This occurred in a specific, hypervariable region of the virus. Interestingly, the specific snippets incorporated kept changing, and multiple variations existed simultaneously.
The researchers observed that viruses with inserted host RNA replicated faster. This suggests that this incorporation might be a key factor in the virus persisting and evading the immune response, potentially contributing to chronicity and failed therapy.
The researchers couldn’t identify specific patterns in the incorporated host RNA, suggesting a random selection process. They theorize that this might create a “race” within the infected person. If HEV incorporates host RNA before the immune system clears the infection, it might establish a chronic foothold.
The presence of host RNA insertions in the viral genome during the acute phase could potentially serve as a biomarker, indicating an increased risk of chronicity. This finding paves the way for further studies in larger patient groups to validate its usefulness as a predictor and potentially guide treatment strategies.
Journal article: Wißing, M.H., 2024. Genetic determinants of host- and virus-derived insertions for hepatitis E virus replication. Nature Communications.
Summary by Stefan Botha










