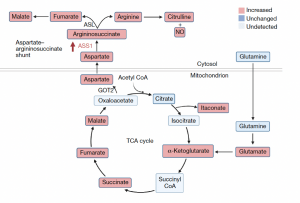
The tricarboxylic acid cycle enzyme fumarate hydratase is involved in the regulation of macrophage cytokine and interferon responses.
A group from the Cologne Excellence Cluster on Cellular Stress Responses in Aging Associated Diseases led by Alexander Hooftman, studied how macrophage fumarate hydratase restrains mtRNA-mediated interferon production (Figure 1). The authors observed a significant increase in fumarate-mediated protein succinate. Mitochondrial membrane potential (MMP) was significantly increased following 48 h of LPS stimulation, but not following 4 h or 24 h of stimulation. Inhibition of the aspartate-argininosuccinate shunt with the glutamic-oxaloacetic transaminase 2 (GOT2) inhibitor aminooxyacetic acid (AOAA) reduced aspartates, asparagine, argininosuccinates and fumarate levels. Silencing of TLR7 or the dsRNA sensors, Ddx58 (which encodes RIG-1) and Ifih (ehich encodes MDA5) reduced IFNβ expression induced by 24 h and 48h of L PS stimulation.
Metabolic rewiring underlies the effector functions of macrophages. Acute fumarate hydratase inhibition suppresses interleukin-10 expression, which leads to increased tumour necrosis factor secretion. The team proposed that the IFN response is driven by cytosolic nucleic acid sensors, such as cGAS, RIG-I or MDA5. In summary, they described a mitochondrial retrograde signalling pathway leading from FH inhibition to mitochondrial membrane hyperpolarization and mtRNA release. Mitochondrial stress may be an underlying mechanism that contributes to type I IFN release in interferonopathies such as systemic lupus erythematosus (SLE). It has previously been demonstrated that PBMCs from patients with SLE have impaired mitochondrial function and altered MMP (Hooftman et al., 2023).
Journal article: Hooftman, A., et al., 2023. Macrophage fumarate hydratase restrains mtRNA-mediated interferon production. Nature.
Summary by Brian Munansangu