In a recent paper, researchers have described a cluster of neurons slow the activity of mice in order to prioritise fighting bacterial infections (Figure 1).
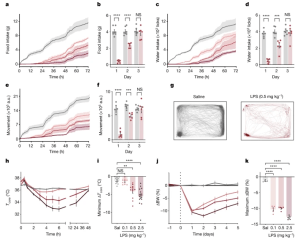
Figure 1: LPS induces coordinated, dose-dependent behavioural and thermal changes. a,c,e, Cumulative food intake (a), water intake (c) and movement (e) for 72 h after injection of saline (grey; N = 7) or LPS at 0.1 mg kg−1 (light red; N = 6), 0.5 mg kg−1 (middle red; N = 6) or 2.5 mg kg−1 (dark red; N = 6). Solid line, mean; shaded area, 95% confidence interval. b,d,f, Food intake (b), water intake (d) and movement (f) for each day post injection with saline (grey) or 0.5 mg kg−1 LPS (red). For all three parameters, there were significant reductions on the first and second days post LPS injection, but full recovery by the third day (two-way ANOVA first day: food intake P < 1 × 10−7, water intake P < 1.6 × 10−6, movement P = 3 × 10−6). g, Movement trace for the first 24 h post saline or LPS (0.5 mg kg−1) for a representative mouse. h, Time course of the core temperature (Tcore) for 48 h after injection of saline (grey; N = 12) or LPS at 0.1 mg kg−1 (light red; N = 11), 0.5 mg kg−1 (middle red; N = 12) or 2.5 mg kg−1 (dark red; N = 15). Points, mean; error bars, s.e.m. i, Minimum change in Tcore for each dose at 48 h post injection (two-way ANOVA: 0.1 mg kg−1 P = 1.0, 0.5 mg kg−1 P = 2.8 × 10−3, 2.5 mg kg−1 P = 4.9 × 10−5; colours as in h; Sal, saline). j, Percentage change in body weight (BW) from day 0 (baseline) for animals injected with saline or LPS (0.1 mg kg−1, n = 6; 0.5 mg kg−1, n = 6; and 2.5 mg kg−1, n = 6; colours as in h) on day 0. k, Maximal percentage decrease in body weight following injection (two-way ANOVA: P < 1 × 10−7 for 0.1 mg kg−1, 0.5 mg kg−1 and 2.5 mg kg−1; colours as in h). **P < 0.01, ***P < 0.001, ****P < 0.0001 and NS, not significant (P > 0.05). All error bars represent the s.e.m.
Through immune response stimulation the researchers were able to show and describe how a specific population of cells found in the brainstem force the body into a “slowed down” in order to battle infection. Through inhibition of these neurons the opposite was observed in that bodily activity resumed as per usual despite infection. These findings have been shown to directly link inflammation to neural pathways regulating behavior, which helps us further our understanding of the relationship between the immune system and the brain
In order to stimulate the immune system and effectively create illness behavior in mice, the research team first exposed the animals to LPS, a component of bacterial cell walls. A group of neurons expressing the neuropeptide ADCYAP1 had an increase after LPS exposure in the dorsal vagal complex, a region of the brainstem. The scientists then turned on those neurons in healthy mice to check that they had discovered the correct brain cells, and they discovered that the animals ate, drank, and walked about less. In contrast, the impact of LPS on these behaviors was much diminished when the ADCYAP1 neurons were silenced.
Journal article: Ilanges, A., et al., 2022. Brainstem ADCYAP1+ neurons control multiple aspects of sickness behaviour. Nature.
Summary by Stefan Botha










