B-cell acute lymphoblastic leukemia (B-ALL) stills remain the first cause of death in childhood around the world. Big advances in therapeutic approaches have improved survival and quality of life in patients. However, relapse events occur often with fatal outcomes. In the era of immunotherapy, the chimeric antigen receptor T (CAR-T) cells and blinatumomab, a bispecific T cell engager, have changed the clinical scenario for evicted B-ALL patients. Therapeutically, CD19 has been an attractive target as is vastly expressed in the majority of B-ALL patients and other lymphoproliferative neoplasms. Therefore, several CAR-T cells and bi-specific engagers have been developed to recognize CD19+ blasts as a target. Nevertheless, since its approval by the U.S. FDA, immunotherapy-resistant patients have been identified basically due the loss expression of the targeted antigen.
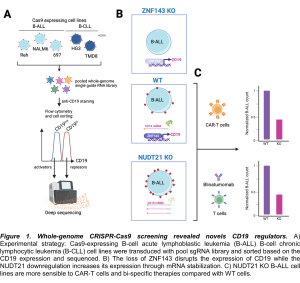
Figure 1. Whole-genome CRISPR-Cas9 screening revealed novels CD19 regulators. A) Experimental strategy: Cas9-expressing B-cell acute lymphoblastic leukemia (B-ALL) B-cell chronic lymphocytic leukemia (B-CLL) cell lines were transduced with pool sgRNA library and sorted based on the CD19 expression and sequenced. B) The loss of ZNF143 disrupts the expression of CD19 while the NUDT21 downregulation increases its expression through mRNA stabilization. C) NUDT21 KO B-ALL cell lines are more sensible to CAR-T cells and bi-specific therapies compared with WT cells.
To rescue the CD19 expression in B-ALL cells and ensure the complete elimination of malignant cells by any of these immunotherapies, Matthew T. Witkowski et al. identified novel regulators by using a surface CD19-guided whole-genome CRISPR-Cas9 screening platform. Thus, three different human B-ALL cell lines (Reh, NALM6 and 697) and two chronic lymphocytic leukemia cell lines (HG3 and TMD8) were used to derivate stable Cas9 expressing cell lines. Consequently, the cell lines were transduced with the Brunello pooled whole-genome single guide RNA library. Based on the expression of CD19, low and high populations were sorted and sequenced. After the gene-set enrichment analysis of the genes that downregulate CD19 (activators), the DNA-binding transcription factor ZNF143 regulates resulted one of the enriched hits across the B-ALL cell lines. In contrast, the RNA-binding protein NUDT21 was identified among the transcription factors that upregulate CD19. Interestingly, when single-cell RNA data were analyzed, the NUDT21 expression correlated with the expression of CD19. Mechanistically, NUDT21 promotes the stabilization of the CD19 mRNA by increasing its polyadenylation and the 3’UTR shortening.
To proof the therapeutic scope, NUTD21 KO B-ALL cells were co-cultured with CAR-T cells by using an organoid-on-a-chip system to mimic the bone marrow microenvironment. Of note, CAR-T cells killed more efficiently the NUTD21 KO cells compared with controls. Similarly, the co-culture of NUTD21 B-ALL cells with peripheral blood CD8+ T cells in the presence of blinatumomab (a CD3-CD19 bi-specific engager) resulted in a higher cytotoxicity.
I believe that CRISPR-Cas9 screening is a powerful tool to reveal novel regulators of current antigens used as a target for immunotherapy. Stabilization of these targets may ensure the therapeutic efficacy and promise to be an important system to guarantee precision oncology (READ MORE).
Journal Article: Witkoski M.T., et al., 2022. NUDT21 limits CD19 levels through alternative mRNA polyadenylation in B cell acute lymphoblastic leukemia. Nature Immunology.
Summary by Juan Carlos Balandrán










