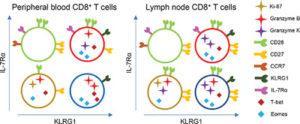
Functional CD8+ T‐cell populations among acute phase effector cells and memory cells can be distinguished by differences in the expression of IL‐7Rα and KLRG1. Cells with a IL‐7Rαlo/KLRG1hi phenotype, previously defined as short‐lived effector cells in mice, persist long after the acute phase of primary CMV infection in humans. (SourceRemmerswaal et al. 2019)
Co-expression of IL-7Rα and KLRG1 in mice can be used to define CD8 effector memory phenotypes during acute viral infections. Early effectors, short-lived effectors and pre-cursor long term memory cells can be defined as IL-7RαloKLRG1lo, IL-7RαloKLRG1hi and IL-7RΑhiKLRG1lo, respectively. Remmerswaal et al., aimed to determine if IL-7Rα/KLRG1 co-expression profiles and functional features of CD8 memory subsets defined in murine studies correspond in persistence and functions to humans memory cells during viral infection.
Researchers studied total and viral-specific CD8 T cells phenotypes in EBV and Human-CMV seropositive healthy adults. Profiling of total non-specific T cells demonstrated that IL-7Rα/KLRG1 co-expression and functions defined in mice, are similar to those observed in humans. Expression of cytotoxic molecules were associated with lack of expression of IL-7Rα either in the presence or absence of KLRG1. Transcriptional profiling of bulk T cells based on IL-7Rα/KLRG1 co-expression patterns showed, that KLRG1 expressing cells were associated with leukocyte activation and immune responses to bacterial antigens, whereas IL-7Rα expressing cells are associated with T cell differentiation. Further suggesting that KLRG1 T cell expression is associated with increased effector functions compared to IL-7Rα expressing T cells. In line with this, they also showed that KLRG1+ CD8 T cells that expressed cytotoxic molecules were predominantly observed in the blood but low frequencies were in the lymph node.
When researchers analysed viral-specific CD8 T cells responses, they observed differential enrichment in IL-7Rα /KLRG1 co-expressing cells depending on the virus. Where, Influenza- and EBV-specific cells were predominantly enriched in IL-7Rα hiKLRG1lo and IL-7Rα hiKLRG1hi , while CMV-specific cells were enriched in IL-7Rα loKLRG1hi only. This phenotype enrichment was associated with reduced expression of Granzyme B and Tbet in influenza- and EBV-specific cells compared with CMV-specific T cells.
In summary, this study highlights the importance of studying antigen-specific memory T cell functional and phenotypic profiles in the context of a specific host and/or infection/disease context. Researchers showed that differentiation models identified in one infection and/or animal model doesn’t apply to all. Where in murine models IL-7Rα loKLRG1hi are short lived effector cells, while in CMV infection this phenotype is also observed in long-term memory and is the predominant memory phenotype associated with CMV-latency.
Article: Remmerswaal et al. 2019. Expression of IL-7Rα and KLRG1 defines functionally distinct CD8+T-cell populations in humans. European Journal of Immunology
Article by Cheleka AM Mpande











