A new study sheds light on how our bodies fight bad cholesterol. It turns out, immune cells in the liver play a crucial role as the body’s first line of defense against excess cholesterol, potentially offering new avenues for preventing heart disease (Figure 1).
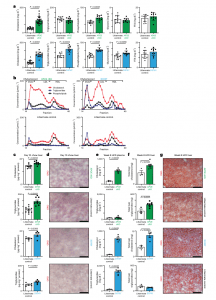
Figure 1: Development of mouse models for inducible APOB lipoprotein dyslipidemia. a, Plasma lipid levels 10 d after tamoxifen administration in APOE cKO (green bars) and D374Y (blue bars) strains together with respective littermate controls in male and female mice fed normal chow (Cholesterol:n = 22 APOE cKO and n = 19 littermate control mice; n = 14 D374Y and n = 19 littermate control mice. Triglycerides: n = 11 APOE cKO and n = 11 littermate control mice; n = 7 D374Y and n = 9 littermate control mice. PLs: n = 10 APOE cKO and n = 11 littermate control mice; n = 9 D374Y and n = 11 littermate control mice. Glycerol: n = 10 APOE cKO and n = 11 littermate control mice; n = 9 D374Y and n = 11 littermate control mice. FFA: n = 10 APOE cKO and n = 11 littermate control mice; n = 9 D374Y and n = 11 littermate control mice). b. Plasma lipoprotein fractionation profiles (μmol L −1) at 10 d after tamoxifen dosing. All curves were calculated as an average of two separately run plasma pools from male and female mice (plasma from 4–6 mice in each pool). c, Cholesterol and triglyceride measurements (μg mg −1 of protein) after liver Folch extraction from male and female mice (Cholesterol: n = 9 APOE cKO and n = 11 littermate control mice; n = 5 D374Y and n = 6 littermate control mice. Triglycerides: n = 9 APOE cKO and n = 11 littermate control mice; n = 5 D374Y and n = 6 littermate control mice). d, Representative pictures of liver section stained with ORO in APOE cKO and D374Y mice with respective littermate controls 10 d after dyslipidemia induction (scale bar, 100 μm). e, Circulating cholesterol and triglycerides (mg dl −1) measured in dyslipidemic APOE cKO, D374Y and respective littermate controls after 8 weeks on an HFD (Cholesterol: n = 5 APOE cKO and n = 3 littermate control mice; n = 5 D374Y and n = 3 littermate control mice. Triglycerides: n = 7 APOE cKO and n = 3 littermate control mice; n = 6 D374Y and n = 3 littermate control mice). f, Hepatic cholesterol and triglyceride levels measured as total liver cholesterol (mg) and total liver triglycerides (mg) in APOE cKO and D374Y with respective littermate controls (Cholesterol: n = 7 APOE cKO and n = 3 littermate control mice; n = 5 D374Y and n = 3 littermate control mice. Triglycerides: n = 7 APOE cKO and n = 3 littermate control mice; n = 5 D374Y and n = 3 littermate control mice). g, ORO representative liver sections of dyslipidemic APOE cKO and D374Y littermate control mice after 8 weeks on an HFD (scale bar, 100 μm). All plots are ±s.e.m. except 1a (±s.d.). For statistical analysis, a two-sided t-test was used (a,c,e,f). FFA, free fatty acid; VLDL, very-low-density lipoprotein.
Cholesterol gets a bad rap, but it’s essential for building healthy cells and hormones. However, high levels of “bad” LDL cholesterol can wreak havoc by clinging to artery walls, forming plaques that narrow blood flow. This build-up, called atherosclerosis, is the main culprit behind heart attacks and strokes, the leading cause of death globally.
Researchers wanted to see how different tissues reacted to a sudden surge of LDL cholesterol. They created a system to quickly increase cholesterol levels in mice.
The researchers were surprised to discover that the liver responded almost instantly, actively removing excess cholesterol. But it wasn’t the usual liver cells acting. Instead, a specialized type of immune cell called Kupffer cells rose to the challenge. Kupffer cells are like the body’s garbage trucks, programmed to identify, and eliminate harmful substances.
The study’s findings were further validated in human tissue samples. This suggests that Kupffer cells play a similar role in our own bodies, highlighting the crucial link between the liver’s immune system and cholesterol regulation. This also opens doors to understanding atherosclerosis as a systemic disease, affecting multiple organs and not just the arteries.
By understanding how the liver and other organs communicate in response to cholesterol overload, researchers hope to develop new strategies to prevent and treat heart disease and related liver problems.
Journal article: Di Nunzio, .G., et al. 2024. Kupffer cells dictate hepatic responses to the atherogenic dyslipidemic insult. Nature Cardiovascular Research.
Summary by Stefan Botha
