Traditionally, blood sugar regulation has been attributed to the hormones insulin and glucagon, produced by the pancreas. Insulin lowers blood glucose by promoting cellular uptake, while glucagon raises it by signalling the liver to release stored glucose. However, researchers suspected that the immune system might also play a role in this essential process.
A recent study has uncovered a surprising role for immune cells in regulating blood sugar levels during periods of low energy, such as fasting or exercise (Figure 1). The research reveals a complex interplay between the nervous, immune, and hormonal systems, with immune cells acting as critical regulators of glucose production. These findings pave the way for novel approaches to managing diabetes, obesity, and even cancer.
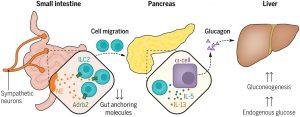
Figure 1: Neuroimmune interactions regulate blood glucose by controlling glucagon secretion. Fasting or high-energy–demand activities lead to low plasma glucose levels that are counter-balanced by glucagon and endogenous glucose production. This work provides evidence that low glucose levels induce intestinal sympathetic neural activity that can promote gut ILC2 migration to the pancreas. Innate type 2 cytokines elicit pancreatic alpha cells to secrete the hormone glucagon, which promotes gluconeogenesis and endogenous glucose production in the liver.
Using genetically engineered mice lacking specific immune cells, the team uncovered a crucial role for a type of immune cell known as ILC2. Mice without ILC2s were unable to produce sufficient glucagon, resulting in dangerously low blood sugar levels during fasting. Remarkably, transplanting ILC2s back into these mice restored normal glucose levels, confirming their role in glucose regulation.
Further investigation revealed that during fasting, ILC2 cells migrate from the intestine to the pancreas. Once there, they release cytokines—chemical messengers that signal pancreatic cells to produce glucagon. This glucagon then prompts the liver to release glucose into the bloodstream, stabilizing energy levels.
The researchers found that this migration of ILC2 cells is directed by the nervous system. Neurons in the gut release chemical signals during fasting, instructing ILC2 cells to move to the pancreas. These signals suppress genes that anchor the immune cells in the intestine, enabling their relocation within hours.
The study sheds light on the metabolic benefits of fasting and exercise. By understanding how immune cells support blood sugar regulation, researchers can better appreciate how these activities contribute to metabolic health.
The discovery has significant implications for managing metabolic and hormonal disorders. Balancing blood sugar is critical not only for preventing obesity but also for addressing the global diabetes epidemic, which affects millions worldwide. Targeting neuroimmune pathways could provide innovative strategies for prevention and treatment.
Journal article: Šestan, M., et al. 2025. Neuronal-ILC2 interactions regulate pancreatic glucagon and glucose homeostasis. Science.
Summary by Stefan Botha










