Chronic hepatitis B (CHB) is a global health concern affecting millions worldwide. This persistent liver infection is characterized by the liver’s inability to clear the hepatitis B virus (HBV), despite the presence of immune cells capable of eliminating infected hepatocytes. A recent study has unveiled a previously unknown mechanism by which the liver actively suppresses immune responses against HBV (Figure 1).
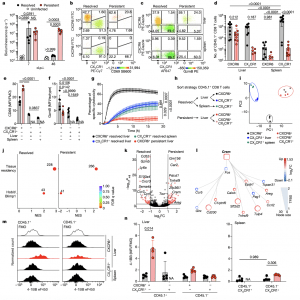
Figure 1: Dysfunctional hepatic virus-specific CXCR6 + CD8 T cells characterized by enhanced CREM activity during persistent hepatotropic infection. a, Liver bioluminescence in vivo imaging of Ad–CMV–GOL (resolved), Ad–TTR–GOL (persistent) infected or uninfected mice. P values determined by one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons per timepoint (n = 5). b,c, Expression of CXCR6, CX 3 CR1 and either CD69 (b) or GzmB (c) by antigen-specific CD45.1+ CD8 T cells in liver and spleen at 45 days post infection (d.p.i.). d–f, Quantification of CXCR6 and CX 3 CR1 (d), CD69 (e) and GzmB (f) expression data from b and c. P values determined by two-way-ANOVA with Tukey’s multiple comparison for adjusted P value (Padj ) (n = 8 (d); n = 5 (e); n = 5 (f)). g, Real-time specific cytotoxicity of CD45.1+ CD8 T cells against OVA 257–264 peptide-loaded hepatocytes. P values determined by one-way ANOVA with Tukey’s multiple comparison of area under the curve (AUC) for Padj (n ≥ 3). h, Scheme of CD45.1+ CD8 T cell FACSorting for RNA-seq analysis. i, Principal component (PC) analysis of RNA-seq results (n = 3). j, GSEA in liver CD45.1+ CXCR6 + CD8 T cells from resolved (left) and persistent (right) infection for a tissue-residency signature and Hobit- and Blimp1-dependent genes (permutation test with Benjamini–Hochberg false discovery rate (FDR)). NES, normalized enrichment score, k, Differentially expressed genes (DEGs) in liver CD45.1+ CXCR6 + CD8 T cells during persistent infection or after resolved infection (red, Padj < 1.31 (P < 0.05 Wald test with Benjamini–Hochberg’s correction) and log 2-transformed fold change (FC)> 1 or >−1, n = 3). l, Transcription factor network analysis comparing CD45.1+ CXCR6 + CD8 T cells in persistent and resolved infection (n = 3). TFBS, transcription factor-binding site. m,n, 4-1BB expression by virus-specific CD45 + CD8 T cells compared to bulk CD45.1 − CD8 T cells at 45 d.p.i. (m) and quantification (n). P values determined by two-way ANOVA with Tukey’s multiple comparison for Padj (n = 5). In a–g,m,n, one out of two or more independent experiments shown; NS, not significant, P ≥ 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Data are mean and s.d. FMO, fluorescence minus one; MFI, geometric mean fluorescence intensity; NA, not analysed.
The liver, the primary target organ of HBV, is a complex microenvironment where immune cells interact with hepatocytes and vascular endothelial cells. While immune cells, particularly cytotoxic T lymphocytes (CTLs), are equipped to eliminate HBV-infected cells, their function is often impaired in CHB. The team discovered that endothelial cells, which line liver blood vessels, play a pivotal role in regulating CTL activity.
Upon entering the liver, CTLs encounter HBV-infected hepatocytes and initiate an immune response. However, prolonged contact between CTLs and endothelial cells triggers a molecular signaling pathway involving cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA). This signaling cascade dampens CTL activity, rendering them less effective in eliminating infected cells. This inhibitory mechanism is thought to be an evolutionary adaptation to protect the liver from excessive immune-mediated damage.
While this protective mechanism is essential for liver homeostasis, it can also contribute to viral persistence in CHB. By prematurely curtailing the CTL response, the liver environment creates a favorable niche for HBV replication. Consequently, chronic inflammation and liver damage ensue.
The identification of this endothelial-mediated immune suppression pathway opens new avenues for therapeutic intervention. Modulating the cAMP-PKA signaling pathway or enhancing CTL resistance to this inhibitory signal could potentially restore effective immune responses against HBV. Furthermore, targeted drug delivery strategies to the liver are crucial to minimize systemic side effects.
Ultimately, a deeper understanding of the complex interplay between the immune system, the liver, and HBV is essential for developing novel immunotherapies to combat CHB. By overcoming the challenges posed by immune suppression, researchers aim to develop strategies that can effectively clear the virus and prevent the progression of liver disease.
Journal article: Bosch, M., et al. 2024. A liver immune rheostat regulates CD8 T cell immunity in chronic HBV infection. Nature.
Summary by Stefan Botha










