Immunotherapy has revolutionised the treatment of oncologic malignancies. Immune checkpoint inhibitors represent a new class of immunotherapy drugs. Professor Elizabeth Quackenbush began her talk by introducing the importance of anti-cancer immune response in protecting the host against primary tumour development or enhancing tumour escape. This process is tightly regulated by immune checkpoints, immune-cell molecules that function as inhibitors (“brakes”) of ongoing immune responses. Checkpoints are usually surface receptors that control either the activation or the inhibition of immune responses. Checkpoint inhibitors that block the checkpoints can restore the body’s anti-cancer mechanisms (e.g. anti-tumour T cells remain activated). Approved checkpoint inhibitors have transformed the cancer treatment landscape and improved the long-term outlook for many patients with late-stage metastatic cancer. But, because of resistance mechanisms, only 25% of patients have positive responses to checkpoint inhibitors.
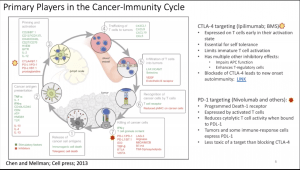
She then described the cancer immunity cycle leading to the accumulation of immune-stimulatory factors that can amplify and broaden T cell responses. This cycle is divided into seven major steps (Figure 1), starting with the release of antigens from the cancer cell and ending with the killing of cancer cells. Prof Quackenbush added that researchers should be aware that T cells trafficking in the blood vessels (step 4) does not always reflect the reality in the tumour (step5). Thus, biomarkers based on immune profiling of blood cells and not the tumour microenvironment could lead you down the wrong rabbit hole.
Each step of the cancer-immunity cycle requires the coordination of numerous factors, both stimulatory and inhibitory in nature. Immune checkpoint proteins, such as CTLA-4, can inhibit the development of an active immune response by acting primarily at the level of T cell development and proliferation (step 3; Figure 1). We distinguish these from immune rheostat (“immunostat”) factors, such as PD-L1 (expressed by activated T cells), which can have an inhibitory function that primarily acts to modulate active immune responses in the tumour microenvironment (step 7). Blocking the binding of PD-L1 to PD-1 (Nivolumab and others) with an immune checkpoint inhibitor (anti-PD-L1 or anti-PD-1) allows the T cells to kill tumour cells.
Prof Quackenbush then reported on the various checkpoint inhibitors currently used and in development. She discussed the antibodies protein targets, binding affinities and the pharmacokinetics measured in various tumour types. She also highlighted new developments and methods to optimize delivery and efficacy in the field of checkpoint inhibitors, including non-fucosylation, prodrug formations, bispecific antibodies, and newer small molecule and peptide checkpoint inhibitors to have better oral bioavailability, fewer immune-related adverse events, improved tumour penetration and a lower production cost.
Prof Quackenbush reported that stromal cells can also co-opt checkpoint molecules to induce cytotoxic T cell apoptosis and ameliorate the CPI efficacy. Indeed, the reduction of macrophage-mediated T cell exclusion increases tumour surveillance by CD8 T cells and renders tumours more responsive to anti–PD-1 treatment in a human lung squam carcinoma reported by Perzanzoni et al.
Several clinical trials have since investigated new checkpoint inhibitors, alone or in combination, for various cancers. This sparked an immense effort to target other immune checkpoint receptors. Upward of 230 clinical trials are underway to evaluate checkpoint inhibitors either as monotherapy or in combination with chemotherapy or targeted therapies. Several novel agents are in early-stage clinical development.
Histologically, tumours can be broadly categorized as:
- Inflamed tumours have high proportions of tumour-infiltrating lymphocytes (TIL), high density of IFNg-producing CD8 T cells, expression of PD-L1 in tumour infiltrating immune cells, possible genomic instability, and the presence of a pre-existing anti-tumour immune response.
- Non-inflamed tumours are immunologically ignorant and are poorly infiltrated by lymphocytes, rarely express PD-L1. Further, proliferating tumours have a low mutational burden and expression of antigen presentation molecules.
Collective clinical evidence to date suggests that checkpoint inhibitors predominantly act by reinvigorating pre-existing anti-tumour T cell responses and are most effective in inflamed tumours. Nevertheless, combination therapy could be the best solution to convert the quiescent immunological profile in non-inflamed tumours to a more inflammatory environment that would respond favourably to checkpoint inhibitors.
This summary gives an overview of some of the topics covered in Professor Queckenbush’s lecture, in our next summary we shall provide an overview of a case study she presented during her talk.
- Interested in learning more about checkpoint inhibitors: read advanced pre-course material on Check-Points Blockade Based Therapies
- Full lecture recording available at IUIS-ALACI-ACAAI Immuno-Colombia 2021 Lecture week 1
Summary by Ichrak Bannour