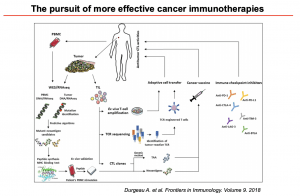
IUIS-ALACI-ACAAI Immuno-Colombia 2021 course took place remotely between 5th to 16th April. The theme of this meeting was “Mechanisms and Approaches to Immunotherapy for Cancer and Chronic Inflammatory Diseases”. This article highlights a talk by Professor Soraya Zorro which focused on Tumour Infiltrating Lymphocytes (TIL) therapy.
IUIS-ALACI-ACAAI Immuno-Colombia 2021 course took place remotely between 5th to 16th April. The theme of this meeting was “Mechanisms and Approaches to Immunotherapy for Cancer and Chronic Inflammatory Diseases”. This article highlights a talk by Professor Soraya Zorro which focused on Tumour Infiltrating Lymphocytes (TIL) therapy.
 In the previous article, we introduced TILs and TIL therapy. In this summary, we shall highlight how cells for TIL therapy are generated, considerations for TIL therapy and utility of co-administration of TIL therapy with other cancer immunotherapies.
In the previous article, we introduced TILs and TIL therapy. In this summary, we shall highlight how cells for TIL therapy are generated, considerations for TIL therapy and utility of co-administration of TIL therapy with other cancer immunotherapies.
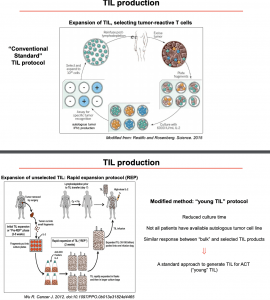
There are two main protocols for TIL production:
- Conventional protocol based on the original protocol designed by Dr. Rosenberg and colleagues, whereby the (i) tumour is isolate, (ii) cells are extracted and cultured ex vivo with IL-2, (iii) selected for tumour recognition, (iv) expanded, and then (v) reinfused post lymphodepletion (Rosenberg S. A., Restifo N. P. 2015). This process selects for mainly CD8+ tumour-specific TILs.
- Rapid expansion protocol also isolates TILs from tumours, however, the process does not select for antigen-specific TILs and has significantly lower culture time than the conventional protocols (Wu R, et al. 2012.).
Current evidence suggests that TIL therapy based on rapid protocol has a better response rate than conventional protocols, potentially due to fewer days in culture resulting in less cell exhaustion. Prof. Zorro also presented data that demonstrated TIL therapy rates of complete and partial responses in melanoma patients (Rosenberg S.A., et al. 2008; Pilon-Thomas S., et al.2012). The advances in TIL therapy has opened doors to treat other types of cancers e.g. cervical cancer, ovarian, pancreatic, and colorectal cancer, with promising results (De Lartigue J. 2020).
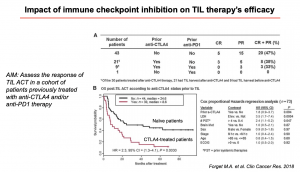 Another interesting point addressed by Prof Zorro was the utility of co-administering TIL therapy with other immunotherapeutic approaches, like checkpoint blockage. The evidence thus far, suggests that patients previously treated with anti-CTLA-4 become more resistant to TILs, which negatively impacts the success rate of TIL and anti-CTLA-4 therapeutic co-treatment (Forget M.A., et al. 2018). Next, Professor Zorro discussed important consideration required when developing the next generation TIL therapeutic products, such as rapid isolation, refined specificity, improved expansion process, enhanced activity, improved persistence, cryopreservation/improved transport and improved safety (available at: https://www.cellulartherapeutics.co.uk/product-development.html). She also highlighted the importance of understanding the underlying mechanisms that impair TIL function such as genetic mutations, antigen presentation, altered trafficking, chronic TCR signalling, immunosuppression and metabolic competition (Anderson K.G., Stromnes I. M., Greenberg P. D. 2017).
Another interesting point addressed by Prof Zorro was the utility of co-administering TIL therapy with other immunotherapeutic approaches, like checkpoint blockage. The evidence thus far, suggests that patients previously treated with anti-CTLA-4 become more resistant to TILs, which negatively impacts the success rate of TIL and anti-CTLA-4 therapeutic co-treatment (Forget M.A., et al. 2018). Next, Professor Zorro discussed important consideration required when developing the next generation TIL therapeutic products, such as rapid isolation, refined specificity, improved expansion process, enhanced activity, improved persistence, cryopreservation/improved transport and improved safety (available at: https://www.cellulartherapeutics.co.uk/product-development.html). She also highlighted the importance of understanding the underlying mechanisms that impair TIL function such as genetic mutations, antigen presentation, altered trafficking, chronic TCR signalling, immunosuppression and metabolic competition (Anderson K.G., Stromnes I. M., Greenberg P. D. 2017).
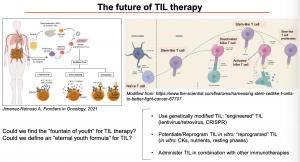 Lastly, Professor Zorro discussed the future of TIL therapy and how to overcome the critical issues in TIL generation, mainly the “fountain of eternal youth” for TILs. This challenge might be resolved with the use of genetically modified TIL (using lentivirus/retrovirus or CRISPR) (Jimenez-Reinoso A., et al. 2021), potentiate or reprogram TIL in vitro (using cytokines and nutrients), and administer TIL in combination with other immunotherapies (Durgeau A., et al. 2018). Improving TIL therapy would be of great use to improve the pursuit of more effective cancer immunotherapies.
Lastly, Professor Zorro discussed the future of TIL therapy and how to overcome the critical issues in TIL generation, mainly the “fountain of eternal youth” for TILs. This challenge might be resolved with the use of genetically modified TIL (using lentivirus/retrovirus or CRISPR) (Jimenez-Reinoso A., et al. 2021), potentiate or reprogram TIL in vitro (using cytokines and nutrients), and administer TIL in combination with other immunotherapies (Durgeau A., et al. 2018). Improving TIL therapy would be of great use to improve the pursuit of more effective cancer immunotherapies.
- Interested in learning more about TILs: read advanced pre-course material on Tumor-infiltrating Lymphocytes (TIL)
- Full lecture recording available at IUIS-ALACI-ACAAI Immuno-Colombia 2021 Lecture week 2
Summary by Carla Sanzochi Fogolin