Introduction to Cutaneous T-cell Lymphoma (CTCL)
Cutaneous T-cell Lymphoma (CTCL) is a rare but clinically significant group of non-Hodgkin lymphomas that primarily manifest in the skin. These lymphomas arise from malignant T-cells that have an affinity for the skin, leading to a variety of presentations, ranging from mild, patchy rashes to severe, tumorous growths. CTCL is often indolent in its early stages, but as it progresses, it can disseminate to lymph nodes, blood, and internal organs, posing significant therapeutic challenges. Understanding the pathogenesis of CTCL has been crucial for developing more effective and targeted treatments. Over the past few decades, there has been substantial progress in elucidating the molecular and immunological underpinnings of CTCL, leading to the development of novel therapeutic strategies (Figure 1).
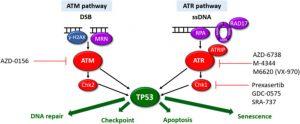
Figure 1: ATM and ATR DDR pathways. The ATM and ATR pathways respond to different types of DNA damage with separate sensors (purple), mediators (blue) and transducers (red). Synthetic lethality can be exploited for cells with aberrant DDR pathways. Cells harbouring LOF mutations in one pathway have increased reliance on the other pathway for DNA damage repair. Inhibition of the intact pathway prevents p53-mediated DNA repair, cell cycle arrest and apoptosis. This results in accumulation of unrepaired DNA damage, mitotic catastrophe triggering p53-independent cell death. Pharmacological agents targeting components of the ATM/ATR pathways currently in clinical development are highlighted.
Pathogenesis and Molecular Insights
The pathogenesis of CTCL is complex, involving multiple genetic, epigenetic, and environmental factors that contribute to the malignant transformation of T-cells. One of the critical insights into the molecular basis of CTCL has been the identification of dysregulated signalling pathways that drive the growth and survival of malignant T-cells. Among these, the aberrant activation of the T-cell receptor (TCR) signalling pathway plays a central role. Mutations in components of the TCR signalling cascade, such as CD28 and PLCG1, have been identified in a significant proportion of CTCL cases, leading to uncontrolled T-cell proliferation and survival.
Additionally, the loss of tumour suppressor genes, such as TP53 and CDKN2A, contributes to the unchecked growth of malignant T-cells. Epigenetic modifications, including DNA methylation and histone acetylation, further exacerbate the malignant phenotype by altering the expression of key regulatory genes. Dysregulation of the JAK/STAT and NF-kB pathways has also been implicated in the pathogenesis of CTCL, providing potential targets for therapeutic intervention. These molecular insights have not only enhanced our understanding of the disease but have also paved the way for the development of targeted therapies that can specifically inhibit these dysregulated pathways, offering hope for more effective treatment options.
Advances in Targeted Therapies
Targeted therapies have revolutionised the treatment landscape for CTCL, offering more effective and less toxic alternatives to conventional chemotherapy. One of the most significant advancements in this area has been the development of brentuximab vedotin, a CD30-targeting antibody-drug conjugate. CD30 is a cell surface marker that is overexpressed in certain subtypes of CTCL, such as mycosis fungoides and Sézary syndrome. Brentuximab vedotin selectively targets CD30-positive cells, delivering a cytotoxic payload that induces cell death while sparing normal cells. Clinical trials have demonstrated the efficacy of brentuximab vedotin in patients with relapsed or refractory CTCL, leading to its approval for use in this patient population.
Another promising targeted therapy is mogamulizumab, an anti-CCR4 monoclonal antibody. CCR4 is a chemokine receptor that is overexpressed on malignant T-cells in CTCL. By binding to CCR4, mogamulizumab depletes malignant T-cells through antibody-dependent cellular cytotoxicity. Mogamulizumab has shown significant activity in patients with advanced CTCL, particularly in those with Sézary syndrome, and has been approved for use in this setting.
In addition to these monoclonal antibodies, small molecule inhibitors targeting specific signalling pathways have shown promise in the treatment of CTCL. For instance, inhibitors of the JAK/STAT pathway, such as ruxolitinib, have demonstrated efficacy in preclinical models and early-phase clinical trials. Similarly, inhibitors of histone deacetylases (HDACs), such as vorinostat and romidepsin, have been approved for the treatment of CTCL based on their ability to induce apoptosis in malignant T-cells by altering the expression of genes involved in cell cycle regulation and apoptosis.
Immunotherapy and Emerging Treatments
Immunotherapy has emerged as a promising treatment modality for CTCL, leveraging the body’s immune system to target and eliminate malignant T-cells. Immune checkpoint inhibitors, such as pembrolizumab, which block the PD-1/PD-L1 pathway, are being investigated for their potential to enhance anti-tumour immune responses in CTCL patients. Early-phase clinical trials have shown encouraging results, with some patients achieving durable responses to treatment. These findings suggest that immune checkpoint inhibitors may offer a new avenue for the treatment of CTCL, particularly in patients who have failed conventional therapies.
Another innovative approach under investigation is adoptive T-cell therapy, which involves the infusion of genetically modified T-cells that are engineered to target specific antigens expressed by malignant T-cells. This approach has shown promise in other types of lymphoma and is now being explored in CTCL. Additionally, therapies targeting epigenetic modifications, such as DNA methylation inhibitors and BET inhibitors, are being studied for their potential to reverse the epigenetic changes that drive CTCL progression.
The tumour microenvironment, which includes immune cells, fibroblasts, and extracellular matrix components, also plays a crucial role in the development and progression of CTCL. Targeting the interactions between malignant T-cells and the tumour microenvironment is an emerging therapeutic strategy. For example, inhibitors of the chemokine receptor CXCR4, which mediates the trafficking of malignant T-cells to the skin, are being evaluated in clinical trials.
Challenges and Future Directions
Despite the progress made in understanding and treating CTCL, several challenges remain. One of the primary challenges is the development of resistance to targeted therapies. Tumour heterogeneity and the ability of malignant T-cells to adapt to therapeutic pressures contribute to the emergence of drug-resistant clones, limiting the long-term efficacy of targeted therapies. To overcome this challenge, researchers are exploring combination therapies that target multiple pathways simultaneously, thereby reducing the likelihood of resistance.
Another challenge is the need for personalised treatment strategies. The molecular and clinical heterogeneity of CTCL means that no single therapy is likely to be effective for all patients. Identifying biomarkers that can predict response to therapy is crucial for the development of personalised treatment plans. Advances in genomic and transcriptomic profiling have the potential to identify such biomarkers and guide the selection of targeted therapies that are tailored to the individual patient’s disease characteristics.
Ongoing research into the mechanisms underlying CTCL, including the role of the tumour microenvironment and immune evasion, will continue to inform the development of new therapeutic strategies. Additionally, the integration of novel therapies, such as immune checkpoint inhibitors and adoptive T-cell therapies, into the treatment paradigm for CTCL offers the potential to improve outcomes for patients with this challenging disease.
Conclusion
Cutaneous T-cell Lymphoma remains a complex and challenging disease to treat, but recent advances in our understanding of its molecular and immunological underpinnings have led to the development of more effective and targeted therapies. The emergence of targeted therapies, such as brentuximab vedotin and mogamulizumab, along with the promise of immunotherapy and novel agents targeting epigenetic modifications and the tumour microenvironment, has transformed the treatment landscape for CTCL. However, challenges such as therapeutic resistance and the need for personalised treatment strategies remain. Continued research into the molecular mechanisms driving CTCL and the development of innovative therapies will be crucial for improving outcomes for patients with this disease.
Journal article: Bakr., F.S., 2022. Advances in the understanding and treatment of Cutaneous T-cell Lymphoma. Frontiers in Oncology.
Summary by Faith Oluwamakinde
References
- Willemze, R., Jaffe, E. S., Burg, G., et al. (2005). WHO-EORTC classification for cutaneous lymphomas. Blood, 105(10), 3768-3785.
- Querfeld, C., Leung, S., Myskowski, P. L., et al. (2012). Primary T-cell lymphomas of the skin. Hematology/Oncology Clinics, 27(1), 129-150.
- Dummer, R., Mihm, M. C., & Burg, G. (2003). Cutaneous T-cell lymphomas: At the crossroads of dermatology and hematology. Archives of Dermatology, 139(7), 896-904.
- Whittaker, S. J., Foss, F. M., & Kashani-Sabet, M. (2006). Treatment of cutaneous T-cell lymphoma. Dermatologic Therapy, 19(2), 101-111.
- Bagot, M., & Sterry, W. (2015). Immunopathogenesis of cutaneous T-cell lymphomas. Hematology/Oncology Clinics of North America, 27(3), 529-548.
- Pileri, A., & Agostinelli, C. (2019). Cutaneous T-cell lymphoma (CTCL): A clinical-pathologic update. Pathology Research International, 2019, 8642806.










