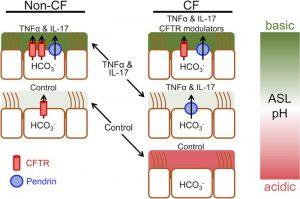
Introduction
Inflammatory cytokines play a pivotal role in the body’s immune response, acting as signalling molecules that regulate inflammation, immunity, and haematopoiesis. Among the myriads of cytokines identified, tumour necrosis factor-alpha (TNF-α) and interleukin-17 (IL-17) are particularly significant due to their involvement in various inflammatory and autoimmune diseases. Their dysregulation has been implicated in the pathogenesis of diseases such as rheumatoid arthritis, psoriasis, and cystic fibrosis. This article delves into the roles of TNF-α and IL-17 in disease progression, focusing on their mechanisms of action, the synergistic effects they produce when acting together, and their potential as therapeutic targets (Figure 1).
TNF-α and Its Role in Disease
TNF-α is a pro-inflammatory cytokine produced by a wide range of cells, including macrophages, dendritic cells, and T cells. It is a critical component of the immune system’s response to infection and injury, primarily due to its ability to induce fever, apoptosis, inflammation, and to inhibit tumorigenesis. TNF-α exerts its effects by binding to its receptors, TNFR1 and TNFR2, which trigger a cascade of intracellular signalling pathways leading to the activation of nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases (MAPKs), and other transcription factors.
In the context of disease, TNF-α is a double-edged sword. While essential for host defence, its overproduction or prolonged activation is detrimental, leading to chronic inflammation and tissue damage. This is evident in rheumatoid arthritis (RA), where elevated levels of TNF-α are found in the synovial fluid and tissues of affected joints. TNF-α promotes the production of other pro-inflammatory cytokines, the recruitment of immune cells, and the destruction of cartilage and bone, perpetuating the inflammatory cycle. Consequently, TNF inhibitors have become a cornerstone in the treatment of RA, demonstrating significant efficacy in reducing symptoms and preventing joint damage.
In addition to RA, TNF-α is implicated in the pathogenesis of psoriasis, a chronic inflammatory skin disease characterized by hyperproliferation of keratinocytes and infiltration of immune cells into the skin. TNF-α stimulates the production of other cytokines, such as IL-6 and IL-8, which contribute to the inflammatory milieu in psoriatic lesions. Anti-TNF therapies have shown considerable success in alleviating the symptoms of psoriasis, further underscoring the central role of TNF-α in disease pathogenesis.
IL-17 and Its Contribution to Inflammatory Diseases
IL-17 is a cytokine primarily produced by Th17 cells, a subset of CD4+ T cells that also produce other pro-inflammatory cytokines like IL-21 and IL-22. IL-17 is crucial in the defence against extracellular pathogens, particularly bacteria and fungi, by promoting the recruitment of neutrophils and the production of antimicrobial peptides. However, similar to TNF-α, dysregulation of IL-17 production or signalling can lead to pathological inflammation.
In autoimmune diseases such as multiple sclerosis, ankylosing spondylitis, and psoriasis, IL-17 is found in elevated levels in affected tissues, where it contributes to the inflammatory process by inducing the expression of pro-inflammatory genes, chemokines, and other cytokines. In psoriasis, IL-17 acts on keratinocytes to produce antimicrobial peptides, chemokines, and cytokines, leading to the recruitment of immune cells and the formation of psoriatic plaques. IL-17 inhibitors, such as secukinumab and ixekizumab, have been developed to block the effects of IL-17, offering significant clinical benefits in patients with moderate to severe psoriasis.
The role of IL-17 in rheumatoid arthritis is also noteworthy. While TNF-α is a key driver of inflammation in RA, IL-17 contributes to the disease by enhancing the production of other pro-inflammatory cytokines and matrix metalloproteinases, leading to joint destruction. Studies have shown that IL-17 can synergize with TNF-α to amplify inflammatory responses, making the targeting of both cytokines a promising strategy in the treatment of RA.
Synergistic Effects of TNF-α and IL-17
TNF-α and IL-17 do not act in isolation; rather, they interact in complex ways that can exacerbate inflammatory responses. This synergism is particularly evident in chronic inflammatory diseases, where the presence of both cytokines leads to a more severe and sustained inflammatory response compared to the action of either cytokine alone. For instance, in rheumatoid arthritis, the combination of TNF-α and IL-17 results in increased production of pro-inflammatory mediators, enhanced recruitment of immune cells, and greater tissue destruction.
The synergistic effects of TNF-α and IL-17 have also been observed in other diseases, such as Crohn’s disease and uveitis. In Crohn’s disease, a chronic inflammatory bowel disease, TNF-α and IL-17 contribute to the inflammatory process by inducing the expression of cytokines, chemokines, and adhesion molecules that promote the infiltration of immune cells into the intestinal mucosa. The combination of TNF inhibitors with IL-17 blockers is being explored as a potential therapeutic approach in patients who do not respond adequately to monotherapy.
In uveitis, an inflammatory disease of the eye, TNF-α and IL-17 are involved in the recruitment and activation of immune cells in the eye, leading to tissue damage and vision loss. The success of TNF inhibitors in treating uveitis suggests that targeting IL-17 may provide additional therapeutic benefits, especially in cases where TNF inhibition alone is insufficient.
Therapeutic Strategies Targeting TNF-α and IL-17
Given their central roles in inflammation and disease pathogenesis, TNF-α and IL-17 have become key targets for therapeutic intervention. TNF inhibitors, such as infliximab, etanercept, and adalimumab, have revolutionised the treatment of rheumatoid arthritis, psoriasis, and other inflammatory diseases. These biologics work by neutralising TNF-α or blocking its receptors, thereby reducing inflammation and preventing tissue damage.
IL-17 inhibitors represent a newer class of biologics that have shown promise in the treatment of psoriasis, ankylosing spondylitis, and other autoimmune diseases. Secukinumab and ixekizumab, both IL-17A inhibitors, have demonstrated significant efficacy in reducing the symptoms of psoriasis, with a favourable safety profile. The development of IL-17 inhibitors has opened new avenues for the treatment of autoimmune diseases, particularly in patients who do not respond to TNF inhibitors.
The combination of TNF-α and IL-17 inhibitors is an area of active research, with the potential to provide more comprehensive control of inflammation in diseases where both cytokines play a role. However, combination therapy also carries an increased risk of infections and other adverse effects, necessitating careful patient selection and monitoring.
Conclusion
The roles of TNF-α and IL-17 in the pathogenesis of inflammatory and autoimmune diseases are well established. These cytokines are not only key mediators of inflammation but also serve as critical targets for therapeutic intervention. The success of TNF inhibitors in treating diseases like rheumatoid arthritis and psoriasis underscores the importance of targeting TNF-α in chronic inflammation. Similarly, the emergence of IL-17 inhibitors has provided new treatment options for patients with autoimmune diseases.
The synergistic effects of TNF-α and IL-17 in promoting inflammation highlight the potential benefits of combination therapy, although this approach must be balanced against the risk of adverse effects. As our understanding of the complex interactions between these cytokines and the immune system deepens, it is likely that more targeted and personalised therapies will emerge, offering hope for patients with chronic inflammatory diseases.
Journal article: Rehman, T., et al., 2021. Inflammatory cytokines TNF-α and IL-17 enhance the efficacy of cystic fibrosis transmembrane conductance regulator modulators. JCI.
Summary by Faith Oluwamakinde
References
- Feldmann M, Maini RN. TNF is defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9(10):1245-1250.
- Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14(9):585-600.
- Kirkham BW, Kavanaugh A, Reich K. IL-17A: a unique pathway in immune-mediated diseases: psoriasis, psoriatic arthritis, and rheumatoid arthritis. Immunology. 2014;141(2):133-142.
- Lubberts E. The IL-17/IL-23 axis in rheumatoid arthritis: a comprehensive review. J Autoimmun. 2008;31(2):124-132.
- McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429-442.