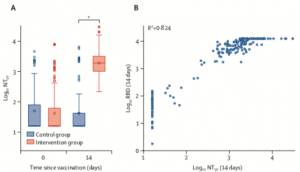
Neutralisation responses
(A) Neutralising antibodies measured in both intervention and control groups on days 0 and 14. (B) Correlation between NT50 and RBD (anti-spike protein) antibody titres. NT50=titres that achieved 50% neutralisation. RBD=receptor-binding domain. *p<0·0001. (Borobia et al. 2021)
There are multiple COVID-19 vaccines that have been approved for (emergency use) including ChAdOx1-S (Oxford AstraZeneca), BNT162b2 (Pfizer-BioNTech), Ad26.COV2.S (Johnson and Johnson), and mRNA-1273 (Moderna), among others. Unfortunately, global access to vaccines is not uniform with some countries having minimal population access to vaccines. Researchers are thus investigating different novel vaccination strategies, such as heterologous prime-boost strategies to facilitate mass COVID-19 vaccinations. There are currently multiple trials that are investigating different strategies such as SARS-CoV-2 adenovirus-based vaccine prime- followed by an mRNA vaccine boost and vice versa. In this summary, we report on findings by Borobia et al., 2021 that performed:
“Phase 2, open-label, randomised, controlled trial on adults aged 18–60 years, vaccinated with a single dose of ChAdOx1-S 8–12 weeks before screening, and no history of SARS-CoV-2 infection. Participants were randomly assigned (2:1) to receive either BNT162b2 (0·3 mL) via a single intramuscular injection (intervention group) or continue observation (control group, vaccinated with ChAdOx1-S).”
The primary outcomes of this study were antibody responses to SARS-CoV-2 spike protein and receptor binding domain, and safety (7-day reactogenicity). Researchers also measure cellular immune responses: SARS-Cov-2-specific IFN-g production using an ELISA assay. Immunogenicity results demonstrated that boost with BNT162b2 induced significantly higher vaccine induced antibody titres, in vitro neutralisation of SARS-CoV-2 and SARS-Cov-2-specific IFN-g production compared to boosting with ChAdOx1-S. The heterologous vaccine regimen was also safe with similar reactogenicity profile as ChAdOx1-S. prime-boost regimen.
Overall, these results demonstrate that BNT162b2 given as a second dose in individuals prime vaccinated with ChAdOx1-S induced a robust immune response, with an acceptable and manageable reactogenicity profile. These results have important implications for future vaccine strategies and potentially promote boosting of adenovirus vaccines with mRNA vaccines to ensure equitable COVID-19 mass vaccination .
Journal Article: Borobia et al.,. 2021. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet
Summary by Cheleka AM Mpande










