In Africa, half a million children succumb to Plasmodium falciparum (the parasite causing malaria) infection each year and our battle against the deadly disease has become a global effort. A recent study from Larsen, et al., published in Nature communications have reported new insights into acquired adaptive immunity towards malaria following infection (Immunity to Malaria). It was reported that the body uses a more efficient mechanism of protection against the parasite when compared to those who have been only vaccinated but never infected by the parasite. Such findings may play an integral role in the development and improvement of existing malaria vaccines. The study by Larsen, et al., has highlighted a significant difference between naturally acquired immunity and vaccine-induced immunity. Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) family members mediate receptor- and tissue-specific sequestration of infected erythrocytes (IEs) in malaria. PfEMP1-specific IgG likely protects by inhibiting IE sequestration and through IgG-Fc Receptor (FcγR) mediated phagocytosis and killing of antibody-opsonized IEs (For more: Malaria Vaccine Breakthrough: Shaking or Stirring the Field?).
It is known that the standard defence against infection from pathogens includes the response from macrophages. In this study, the researchers have identified and described how the immune response and immunity to malaria seems to work differently. It appears that the immune system acts via a different mechanism to combat malaria infection. They described the difference in antibody phenotype which is dependent of immunisation via vaccine or previous infection. Antibody responses are a central component of naturally acquired malaria immunity. This points to the reliance on Natural killer cells to rid the body of this deadly parasite. It seems that the bodies response to malaria shares similarities to the body’s immune response against cancer. It can be said that the immune system has a somewhat “tailored” defence against malaria. Larson, et al., reported that antibody responses to P. falciparum antigens expressed on the IE surface are also a subject to afucosylation. Additionaly highlighting that naturally acquired IgG responses to the PfEMP1 antigens VAR6 and VAR2CSA as well as its absences in responses to the merozoite antigen GLURP and VAR2CSA-specific IgG induced by subunit vaccination. To summarise the patient cohort, blood samples from Ghanaian participants, infected with malaria were compared to uninfected participants and with blood samples from people who participated in Phase 1 clinical trials of an experimental malaria vaccine. They reported the affinity of afucosylated IgG to FcγRIIIa is being up 40-fold higher than fucosylated IgG, which resulted in enhanced antibody-dependent cellular cytotoxicity. Interestingly, it is know that the majority of IgG in plasma is fully fucosylated, but afucosylated IgG is was seen in a response to enveloped viruses and to paternal alloantigens highlighted during pregnancy. Naturally acquired PfEMP1-specific IgG is strongly afucosylated in a stable and exposure-dependent manner, and efficiently induces FcγRIIIa-dependent natural killer (NK) cell degranulation (Figure 1). In contrast, immunization with a subunit PfEMP1 (VAR2CSA) vaccine results in fully fucosylated specific IgG (Figure 2).
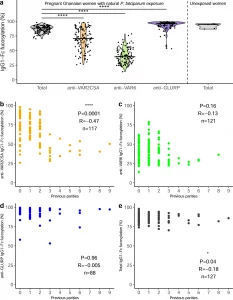
Figure 1: Fc fucosylation of naturally acquired P. falciparum-specific IgG depends on antigen location and exposure. a Fc fucosylation levels of total plasma IgG1 (gray, n = 127) and IgG1 specific for VAR2CSA (orange, n = 117), VAR6 (green, n = 121), and GLURP (blue, n = 88) in Ghanaian pregnant women (left four panels). Fc fucosylation levels of total plasma IgG1 from unexposed Dutch women (n = 5) were included for comparison (right panel). Medians and densities are shown. Statistically significant pairwise differences between antigen-specific IgG and total IgG (multiple two-sided Wilcoxon signed-rank tests with Bonferroni correction) are indicated (****P < 0.0001). b–e Correlations of b VAR2CSA-, c VAR6-, d GLURP-specific, and e total IgG1-Fc fucosylation levels with parity. P values and correlation coefficients are shown. Statistical significance of correlations (Spearman’s correlations. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (Larson, et al., 2021).
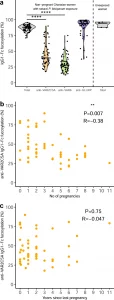
Figure 2: Fc fucosylation levels of VAR2CSA-specifc IgG is temporally stable. a Fc fucosylation levels of total plasma IgG1 (gray, n = 72) and IgG1 with specificity for VAR2CSA (orange, n = 50), VAR6 (green, n = 65), and GLURP (blue, n = 43) in non-pregnant Ghanaian women exposed to VAR2CSA during one or more previous pregnancies. Fc fucosylation levels of total plasma IgG1 from unexposed Dutch females (n = 5) are included as controls. Medians and densities are shown. Statistically significant pairwise differences between antigen-specific IgG and total IgG (multiple two-sided Wilcoxon signed-rank tests with Bonferroni correction) are indicated (****P < 0.0001). b Correlation between fucosylation levels of VAR2CSA-specific IgG1 and parity. c Correlation between fucosylation levels of VAR2CSA-specific IgG1 and time since last pregnancy. Statistical significance of correlations are shown (Spearman’s correlations. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001) (Larson, et al., 2021).
In their own words:
“Our study supports the hypothesis that the immune system has evolved a capacity to selectively modulate the glycosylation pattern of the IgG Fc region, thereby fine-tuning the effector response triggered by antibody-opsonized targets.”
More on Immunopaedia – Malaria Vaccines- Recent Advances and New Horizons
Journal article: Larsen, et al., 2021. Afucosylated Plasmodium falciparum-specific IgG is induced by infection but not by subunit vaccination. Nature Communications.
Summary by Stefan Botha
