The hypoxia-inducible factors (HIFs) regulate the main transcriptional pathway of response to hypoxia in T cells and are negatively regulated by von Hippel-Lindau factor (VHL). The role of HIFs in the regulation of CD4 T cell responses during infection with M. tuberculosis isn’t well understood.
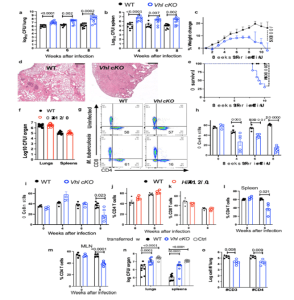
Figure 1: VHL expression in T cells is critical for the control of M. tuberculosisinfection in mice a,b, Vhl cKO and WT mice were infected via aerosol with 250 M. tuberculosis and sacrificed at indicated time points after infection. The log10 CFU in the lung (a) and spleen (b) of individual mice (n WT = 10, 9, 12; n Vhl cKO = 9, 9, 10 mice at 4-, 6- and 8-weeks post infection (w.p.i.) respectively) are represented. cThe weight change with respect to the uninfected group mean (day 0) during infection with M. tuberculosis of Vhl cKO and WT mice (n = 11 per group) is depicted. D Representative micrographs from hematoxylin-eosin-stained paraffin lung sections from Vhl cKO and WT mice 8 w.p.i. with M. tuberculosis (bar: 400 μm). E The cumulative mortality of Vhl cKO and WT mice (n = 9 per group) after M. tuberculosis infection is depicted. F The log10 CFU in the lungs and spleens of Hif1a cKO (n = 11) and WT (n = 9) mice 8 w.p.i. with M. tuberculosis are shown. g–I The representative dot plots of CD4 and CD8 (gated on T cells) in the lungs of Vhl cKO and WT mice before and 8 w.p.i. with M. tuberculosis (g), and the frequency of CD4 (h) and CD8 (i) T cells in the lungs (WT n = 4, 4, 6, 8 and Vhl cKO n = 4, 5, 6 and 8 mice at 0, 4, 6 and 8 w.p.i. respectively). j, k The frequency of CD4 and CD8 T cells in the lungs of Hif1a cKO and WT mice before (n = 4 per group) and 8 w.p.i. (n = 5 per group) are depicted. l, m The frequency of CD4 in spleens (l) and mediastinal lymph nodes (m) before and 8 w.p.i. with M. tuberculosis are shown (Vhl cKO n = 4, 7 and WT n = 4, 4 mice at 0 and 8 w.p.i., respectively). Rag2−/−mice were administered i.v. with either 2.106 Vhl cKO or WT CD4 T cells (n = 6) or left untreated 3 days after M. tuberculosis infection. The log10 CFU in the lungs and spleens (n) (n = 8 animals per group) and number of CD4 T cells (o) in lungs (n = 6 mice per group) 4 w.p.i. Each symbol represents one mouse, and the data are presented as the mean ± s.e.m. The p-values were calculated using either a two-tailed unpaired t test with Welch’s correction for no homoscedasticity and FDR approach for multiple comparison (a,b,f,h–m,o), a one-way ANOVA with Welch’s correction (n), a 2-way ANOVA with Sidak’s multiple comparison test (c), or a χ2 test (e). Source data are provided as a Source Data file.
The authors show that mice lacking VHL in T cells (Vhl cKO) are highly susceptible to infection with M. tuberculosis, which is associated with a low accumulation of mycobacteria-specific T cells in the lungs that display reduced proliferation, altered differentiation and enhanced expression of inhibitory receptors. In contrast, the absence of HIF-1 in T cells is redundant in M. tuberculosis control. Vhl cKO mice also exhibit diminished immunisation responses. Moreover, upon TCR activation, VHL promotes appropriate MYC-activation, cell-growth responses, DNA synthesis, proliferation, and survival of CD4 T+ cells. The VHL-deficient T cell responses are rescued by the lack of HIF-1, demonstrating that the enhanced susceptibility to M. tuberculosis is mediated by HIF-1. HIF-1 is required for tuberculosis infection and the poor responses of Vhl-deficient T cells.
Journal article: Liu, R., et al., 2022. HIF-1 stabilization in T cells hampers the control of Mycobacterium tuberculosis infection. Nature communications.
Summary by Brian Munansangu










