Stroke is a complex condition with acute neurological consequences and potentially long-term systemic implications. While its impact on the brain is well-established, the organ-wide repercussions remain an understudied area.
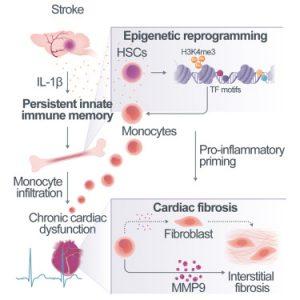
In a recent study, researchers investigated the elevated incidence of comorbidities post-stroke and if they might share a common immunological root (Figure 1). The research team utilized single cell sequencing to uncover persistent proinflammatory alterations in the transcriptome of specific immune cells (monocytes/macrophages) across multiple organs. These epigenetic modifications, characterized by altered gene expression, disrupt the proteome and are particularly pronounced in the heart, where they contribute to scarring and impaired cardiac function.
Identifying interleukin-1β (IL-1β) as a key driver of these post-stroke epigenetic changes, the researchers demonstrated in a murine model that excessive IL-1β in the bone marrow induces cardiac dysfunction. Moreover, blocking IL-1β and inhibiting the migration of proinflammatory cells to the heart effectively prevented cardiac complications.
These findings offer a novel perspective on the pathophysiology of post-stroke comorbidities, suggesting that the epigenetic reprogramming of the immune system within the brain-heart axis represents a potential therapeutic target for preventing secondary cardiac conditions.
Journal article: Alba Simats, A., et al., 2024. Innate immune memory after brain injury drives inflammatory cardiac dysfunction. Cell.
Summary by Stefan Botha