In a recent study, a group of researchers revealed a protein that is frequently present in lung cancer cells at high levels and that regulates a crucial immunosuppressive pathway that enables lung tumours to avoid immune response (Figure 1). The finding could speed the creation of therapies that defeat this tumour defence mechanism and enhance patient outcomes in lung cancer cases.
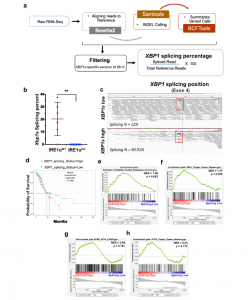
Figure 1: XBP1s is associated with decreased overall survival in human NSCLC. a Schematic of the RNA-seq based XBP1s detection pipeline. b Box and whisker plot of computationally evaluated Xbp1 splicing in isogenic IRE1αWT (red, n = 6) or IRE1αKO (blue, n = 10) HKP1 cancer cells. Data were presented as mean ± SD. P < 0.0002. Unpaired, two-tailed, Student’s t-test. c Visual survey of aligned reads using Integrative Genomic Viewer (IGV) showing detected indels including the 25 nucleotide excision of the XBP1 gene (red box). Representative XBP1s low and XBP1s high samples are shown. d Kaplan–Meier survival plots depicting associations between overall survival (OS) and XBP1s status in human TCGA-LUAD. High is top 1/3rd (n = 103) and low is bottom 1/3rd (103) of the XBP1splicing scores. The high XBP1s group is the reference population. HR is the hazard ratio and p adj is the log-rank p value from the multivariate Cox proportional-hazards regression model adjusted for age at diagnosis, gender, pathology, TN stages, and smoking history. e–h GSEA hyperparametric curves showing expression of genes controlled by the UPR (e), XBP1s (f), PERK (g), and ATF6 (h) in patients with high vs. low XBP1s levels. Source data are provided as a Source Data file
The study’s findings, which were supported by analyses of human lung cancer datasets and tests in preclinical lung cancer models, demonstrate that the transcription factor XBP1s promotes tumour survival by inhibiting the anti-tumor activity of nearby immune cells. They found that the synthesis of the potent immunosuppressive chemical prostaglandin E2 is how XBP1s causes this impact.
The unfolded protein response pathway, which is persistently elevated in many malignancies, was the focus of the study’s attention. It consists of the IRE1-XBP1 arm. Other tumour types have been the subject of investigations in the past, and it has been demonstrated that this route not only directly encourages the survival and growth of tumours but also works to limit the antitumor activity of adjacent immune cells.
The gene activity “signature” resulting from IRE1 knockout in mouse non-small cell lung cancer (NSCLC) tumours was also mapped as part of the work. They discovered that this gene signature’s presence in human NSCLC tumours predicted improved patient survival.
They suggested that in the future, a clinical test based on this signature would be helpful for prognosticating outcomes and choosing the best therapies.
Journal article: Crowley, M.J.P., et al., 2023. Tumour-intrinsic IRE1α signaling controls protective immunity in lung cancer. Nature Communications.
Summary by Stefan Botha










