Osteoporosis, a condition affecting over 200 million people globally, is characterized by a loss of bone mineral density (BMD) and changes in bone microstructure. This makes bones more fragile and prone to fractures. Traditionally, osteoporosis was understood as a metabolic disease influenced by hormones and mechanical factors. However, emerging research in osteoimmunology— the study of the interaction between the immune system and bone—reveals the role of immune molecules like interferon-gamma (IFN-γ) in bone re-modelling. A recent review published in Frontiers in Immunology explores how IFN-γ influences the balance between bone formation and resorption, offering new insights for osteoporosis treatment.
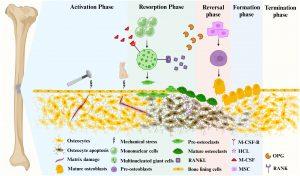
Figure 1: Bone remodeling consists of 5 major distinct but overlapping phases. Mature osteoblasts are generated by the differentiation of mesenchymal stem cells into pre-osteoblasts. Mature osteoclasts arise from multinucleated giant cells differentiated from monocytes stimulated by two important factors, M-CSF and RANKL, followed by differentiation into pre-osteoclasts, and finally into mature osteoclasts. Phase 1 (Activation Phase): Initiating or activating bone remodeling at a specified area. Phase 2 (Resorption Phase): Osteoprogenitors and mesenchymal stem cells are simultaneously recruited while bone resorption occurs. Phase 3 (Reversal Phase): The bone remodeling process shifts from bone resorption to bone formation. Phase 4 (Formation phase): completing bone remodeling and osteoid mineralization. Phase 5 (Termination Phase): New bone replaces old bone to retain bone strength and quality. Maintaining an environment of resting bone surfaces until the next bone remodeling begins.
Bone Remodelling and IFN-γ
Bone remodelling is a continuous process where osteoclasts (OCs) break down old bone, and osteoblasts (OBs) build new bone. This balance is crucial for maintaining bone strength. Osteoporosis occurs when bone resorption surpasses bone formation. IFN-γ, a cytokine produced by immune cells, is found to regulate this balance by influencing both OB and OC activity.
The effects of IFN-γ are complex and vary depending on the cellular environment, dose, and timing of exposure. In some contexts, IFN-γ inhibits osteoclastogenesis (the formation of OCs), reducing bone resorption. However, in other conditions, particularly in inflammatory environments, IFN-γ can promote OC activity, leading to bone loss.
Key Findings on Osteoclasts and Osteoblasts
IFN-γ’s role in osteoclasts (cells that break down bone) has been widely studied. The cytokine inhibits OC formation by degrading TRAF6, a key signalling molecule required for OC differentiation. By blocking this pathway, IFN-γ reduces OC maturation and activity, leading to decreased bone resorption.
Conversely, IFN-γ’s effect on osteoblasts (cells that form bone) is two-fold. At early stages, IFN-γ inhibits OB differentiation by suppressing key osteogenic factors like Runx2, which is essential for bone formation. However, at later stages, IFN-γ can stimulate OB activity, promoting bone formation through the Wnt/β-catenin signalling pathway. This dual role of IFN-γ highlights its potential as both a therapeutic target and a treatment strategy for osteoporosis.
Clinical Implications
Understanding the role of IFN-γ in osteoporosis opens new doors for therapeutic interventions. The paradoxical nature of IFN-γ—being both protective and destructive in different contexts—suggests that its clinical application needs to be carefully controlled. For example, using IFN-γ to inhibit OC activity could be beneficial in reducing bone loss in postmenopausal osteoporosis. On the other hand, the cytokine’s bone-resorptive effects must be mitigated in inflammatory conditions like rheumatoid arthritis, where bone loss is driven by excessive immune activation.
Future treatments may involve selectively modulating IFN-γ activity, either by enhancing its protective role in bone formation or by inhibiting its destructive effects on bone resorption. Additionally, understanding how IFN-γ interacts with other cytokines, such as TNF-α and RANKL, which are also involved in bone re-modelling, could lead to more targeted and effective treatments for osteoporosis and other bone-related diseases.
The review highlights IFN-γ’s complex and context-dependent role in bone re-modelling, offering valuable insights into osteoporosis treatment. While IFN-γ can inhibit bone resorption, it also has the potential to promote bone loss under certain conditions. Future research should focus on harnessing IFN-γ’s beneficial effects while minimizing its negative impact, paving the way for new therapeutic strategies in osteoimmunology.
Journal Article: Li, Siying, et al. “Osteoporosis: Interferon-Gamma-Mediated Bone Remodeling in Osteoimmunology.” Frontiers in Immunology.
Summary by Faith Oluwamakinde










