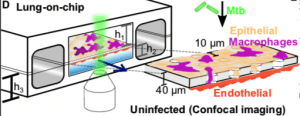
Schematic of the LoC model of early tuberculosis. Confluent layers of alveolar epithelial cells AECs) and endothelial cells populate the top and bottom faces of the porous membrane that separates the air-filled “alveolar” (upper) and liquid-filled “vascular” (lower) compartments, creating an air-liquid interface (ALI). GFP-expressing macrophages (magenta) are added to the alveolar compartment to mimic the natural route of infection. h1 = 1 mm, h2 = 250 μm, h3 = 800 μm. (Source: Thacker et al., 2020)
One of the caveats of in vitro studies is the inability to model the complexities associated with a whole organ. In response to this limitation, the organ-on-a-chip technology which combines microchip technology with cell culture enabling tissue and organ functionality was developed. We have previously reported on the use of this tech to gain a better understanding of the impact mechanistic breathing has on the growth of lung cancer in the alveoli and airway (Read more: Lung on a chip model recapitulates lung cancer pathology and therapy). In this article, we highlight a recently published study by Thacker et al., 2020, which utilised the lung-on-a-chip to understand the role of surfactants during the early stages of Mycobacterium tuberculosis (M.tb) growth.
“We used our model to observe where the sites of first contact are, how M. tuberculosis grows in alveolar epithelial cells compared to bacteria-killing cells called macrophages, and how the production of surfactant affects growth, all while maintaining these cells at the air-liquid interface found in the lung.” Source: eLife BlogPost
Researchers chose to use murine over human cells to seed the lung-on-a-chip model because they wanted to track macrophages (that constitutively expressed green fluorescent protein) without the need for additional markers. They also wanted to compare results from their with results from previously reported murine M.tb models. Using their model they showed that surfactants play an essential role in restricting bacterial group. They showed that in the absence of surfactants (a condition that can not be modelled in vivo due to its lethality) M.tb grows optimally and the addition of exogenous surfactants restores cellular control of M.tb. They also demonstrated “the surfactant-mediated removal of these virulence-associated lipids, thus suggesting an additional mechanism for the attenuation of intracellular growth of M.tb in macrophages.”
Authors of the study concluded “that their findings suggest a potential role for pulmonary surfactant replacement formulations in host-directed therapies against tuberculosis. These insights were made possible by use of an organ-on-chip system that reproduces host physiology in a modular and tuneable fashion, which is frequently impossible to achieve in vivo.”
Most importantly this article demonstrates the utility of the lung-on-a-chip technology to study immunity associated with M.tb infection without the need for animal models.Schematic of the LoC model of early tuberculosis. Confluent layers of AECs and endothelial cells populate the top and bottom faces of the porous membrane that separates the air-filled “alveolar” (upper) and liquid-filled “vascular” (lower) compartments, creating an air-liquid interface (ALI). GFP-expressing macrophages (magenta) are added to the alveolar compartment to mimic the natural route of infection. h1 = 1 mm, h2 = 250 μm, h3 = 800 μm.
Journal Article: Thacker et al., 2020. A lung-on-chip model of early M. tuberculosis infection reveals an essential role for alveolar epithelial cells in controlling bacterial growth. eLIFE
Article by Cheleka Mpande










