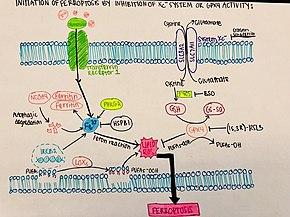
Rheumatoid arthritis (RA) is a complex autoimmune disease characterized by chronic joint inflammation and progressive joint damage. While inflammation is a hallmark of RA, the role of iron overload and ferroptosis in this process is only beginning to be understood. A 2024 study published in Redox Biology explores how focal iron overload in macrophages exacerbates RA through a process known as ferroptosis—an iron-dependent form of cell death.
Study Overview and Findings
The researchers examined macrophages from the synovial fluid of RA patients, finding that macrophages exhibited varying susceptibility to ferroptosis based on their iron content. Macrophages are immune cells that play dual roles in inflammation; M1 macrophages are pro-inflammatory, while M2 macrophages are anti-inflammatory. The study revealed that while M2 macrophages are more prone to iron-induced ferroptosis, M1 macrophages are relatively resistant.
Ferroptosis is driven by iron-induced lipid peroxidation, which damages cellular membranes. In RA, iron accumulates in the joints, leading to an imbalance between pro- and anti-inflammatory macrophages. M2 macrophages, which usually help resolve inflammation, are destroyed by ferroptosis, worsening the disease by allowing M1 macrophages to dominate the inflammatory environment. The study also showed that the loss of glutathione peroxidase 4 (GPX4), a key enzyme in protecting cells from ferroptosis, was a major factor in this process.
Key Experimental Results
The research team used a mouse model of RA, inducing arthritis through serum transfer. Treatment with a ferroptosis inhibitor, liproxstatin-1 (LPX-1), significantly reduced joint swelling and inflammation. The inhibition of ferroptosis not only reduced the severity of arthritis but also restored the balance between M1 and M2 macrophages in the joints.
Further experiments revealed that iron overload led to increased lipid peroxidation and cell death in M2 macrophages, but not in M1 macrophages. This differential susceptibility suggests that targeting iron metabolism in macrophages could be a novel therapeutic strategy for RA.
Implications for RA Treatment
The findings of this study suggest that managing iron overload and preventing ferroptosis in M2 macrophages could offer a new approach to treating RA. By preserving the anti-inflammatory functions of M2 macrophages, it may be possible to control chronic inflammation and slow the progression of joint damage.
Iron chelation therapy, which reduces excess iron levels in the body, could also be explored as a complementary treatment for RA. While previous studies showed mixed results with iron chelation, this new research highlights the importance of targeting specific immune cell populations, like macrophages, for better therapeutic outcomes.
This study identifies ferroptosis as a key mechanism in the progression of RA, particularly through the destruction of anti-inflammatory M2 macrophages. By targeting iron overload and preventing ferroptosis, new treatment strategies for RA could emerge, offering hope for better management of this debilitating disease.
Journal Article: Liu, Yan, et al. “Heterogeneous Ferroptosis Susceptibility of Macrophages Caused by Focal Iron Overload Exacerbates Rheumatoid Arthritis.” Redox Biology.
Summary by Faith Oluwamakinde