In a recent study published in Signal Transduction and Targeted Therapy, researchers have unveiled a novel approach to enhance the efficacy of CD8+ T cell-based immunotherapies against cancer (Figure 1). The team identified that overexpressing the protein LIM-domain-only 4 (LMO4) in CD8+ T cells bolsters their stem-like properties, leading to improved tumor rejection. This discovery offers promising avenues for advancing treatments for solid tumors, which have historically been challenging to address with existing immunotherapies.
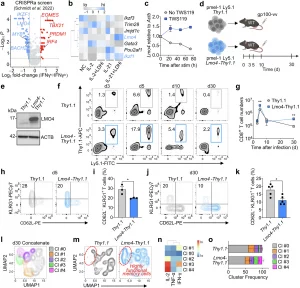
Figure 1: Lmo4 overexpression enhances CD8+ T cell stemness and polyfunctionality. a Volcano plot displaying median sgRNA log2-fold change (IFN-γhi/lo sorting bin counts) for each gene tested in the genome-wide CRISPRa screen.17 Genes included in the analysis were exclusively transcription factors or transcription regulators. b Heatmap depicting the expression levels (assessed by RNA-seq) of the top negative hits identified in the CRISPRa screen in CD8+ T cells cultured for 4 d under different conditions: No Cytokine (NC), IL-2, IL-2 + LDHi, IL-21, IL-21 + LDHi23. c q-PCR of Lmo4 mRNA in CD8+ T cells cultured for 72 h with or without TWS119. d Experimental design to assess the impact of Lmo4 overexpression on stem-like CD8+ T cell formation. e Immunoblot of LMO4 in Thy1.1 and Lmo4-Thy1.1 overexpressing T cells. ACTB served as control. f, g Flow cytometry analysis (f) and quantification (g) of splenic pmel-1 CD8+ T cells following transfer of either 105 pmel-1 Ly5.1+ Thy1.1+ or Lmo4-Thy1.1+ CD8+ T cells into wild-type mice infected with gp100-vv. Assessment was conducted at various time points from 3 to 30 days post-transfer, with three mice per group for each time point. Data are from one representative of 3 experiments. h, i Flow cytometry analysis (h) and percentages (i) of CD62L−KLRG1+ splenic pmel-1 T cells 5 d after transfer as in (f, g). j, k Flow cytometry analysis (j) and percentages (k) of CD62L−KLRG1+ splenic pmel-1 T cells 30 d after transfer as in (f, g). l UMAP plot of concatenated Thy1.1+ and Lmo4-Thy1.1+ pmel-1 CD8+ T cells isolated from spleens 5 d after treatment as in (f, g) showing the distribution of clusters (Cl) identified by FlowSOM. m UMAP plot of concatenated Thy1.1+ and Lmo4-Thy1.1+ pmel-1 CD8+ T cells showing differences in cluster distributions. n Heatmap showing the relative expression levels of indicated cytokines in the FlowSOM clusters. o Bar plot of Thy1.1+ and Lmo4-Thy1.1+ pmel-1 CD8+ T cells isolated from spleens 5 d after treatment as in (f, g) quantifying the distribution of clusters assessed by FlowSOM. *P < 0.05, **P < 0.01 (unpaired two-tailed Student’s t-test). (d) was created with BioRender.com
The Quest for Potent T Cell Therapies
CD8+ T cells are pivotal in the body’s defense against tumors. Their effectiveness in cancer therapy is closely linked to their ability to maintain a stem-like state, characterized by self-renewal and the capacity to differentiate into various functional states. High frequencies of these stem-like memory T cells in therapeutic infusions have correlated with superior patient outcomes across multiple clinical trials. However, a significant challenge has been the tendency of these cells to undergo terminal differentiation, losing their stem-like qualities and, consequently, their therapeutic potency.
Unveiling LMO4’s Role
To address this challenge, the research team analyzed data from a published CRISPR activation screen, aiming to identify transcriptional regulators that could enhance stem-like behavior in CD8+ T cells. Among the top candidates was LMO4, a member of the LIM-domain-only protein family known for its role in regulating cell fate and differentiation. Interestingly, LMO4 was found to be downregulated upon CD8+ T cell activation but maintained under culture conditions that promote the formation of stem-like T cells.
Engineering Superior T Cells
Employing a synthetic biology approach, the researchers ectopically expressed LMO4 in antitumor CD8+ T cells. This genetic modification led to a selective expansion and enhanced persistence of the modified cells, while limiting their terminal differentiation and senescence. The LMO4-overexpressing T cells exhibited increased expression of genes associated with stemness, such as Tcf7, Socs3, Junb, and Zfp36, which are crucial for memory responses.
Enhanced Antitumor Activity
When tested in both syngeneic and xenograft tumor models, the LMO4-overexpressing CD8+ T cells demonstrated superior antitumor immunity, resulting in enhanced tumor regression. This effect was attributed to the cells’ increased polyfunctionality and recall capacity, essential features for effective and sustained antitumor responses.
Mechanistic Insights
Further investigation revealed that LMO4 does not directly modulate gene transcription. Instead, it binds to Janus kinase 1 (JAK1) and potentiates Signal Transducer and Activator of Transcription 3 (STAT3) signaling in response to interleukin-21 (IL-21). This interaction enhances the expression of target genes vital for maintaining T cell stemness. Notably, deleting Stat3 using CRISPR/Cas9 technology nullified the enhanced memory signature conferred by LMO4, thereby abrogating the therapeutic benefit of LMO4 overexpression.
Implications for Immunotherapy
These findings establish LMO4 overexpression as an effective strategy to boost CD8+ T cell stemness, providing a new synthetic biology tool to bolster the efficacy of T cell-based immunotherapies. By enhancing the stem-like properties of CD8+ T cells, this approach holds the potential to improve outcomes for patients undergoing T cell-based treatments, particularly in the context of solid tumors where current therapies often fall short.
Future Directions
The study opens avenues for further research into the application of LMO4 overexpression in clinical settings. Future studies will be necessary to assess the safety and efficacy of this approach in humans, as well as to explore the potential for combining LMO4 overexpression with other therapeutic strategies to maximize antitumor responses.
In conclusion, the overexpression of LMO4 represents a promising advancement in the field of cancer immunotherapy, offering a novel method to enhance the stem-like properties of CD8+ T cells and improve their capacity to combat tumors.
Journal article: Schelker, R.C., et al., 2024. LIM-domain-only 4 (LMO4) enhances CD8+ T-cell stemness and tumor rejection by boosting IL-21-STAT3 signaling. Sig Transduct Target Ther.










