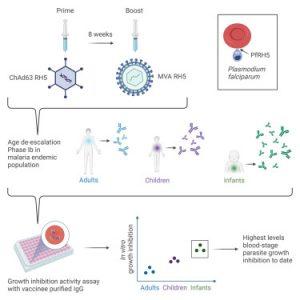
In a new study, researchers have presented data from a malaria vaccine demonstrating both safety and the capacity to induce an immune response in African infants, a demographic highly vulnerable to severe malaria (Figure 1).
This vaccine targets a protein called RH5, which the malaria-causing parasite Plasmodium falciparum employs to invade red blood cells. The researchers found that this approach generates a robust immune response, particularly among infants, in whom malaria poses a significant threat.
Most existing malaria vaccines, including RTS,S, focus on training the immune system to target the malaria parasite at its sporozoite stage, before it infiltrates the liver. However, RH5 is activated when the parasite emerges from the liver, infects red blood cells, and triggers the disease.
This study revealed that the vaccine can safely elicit substantial anti-RH5 immune responses in infants from malaria-endemic areas. Moreover, if an anti-sporozoite vaccine and an anti-RH5 vaccine were used in combination in the future, it could potentially offer more comprehensive and prolonged protection against malaria.
While the trial established the vaccine’s safety in a malaria-endemic population, participants reported only mild and transient side effects like pain at the injection site and a slight fever. Additionally, the vaccine prompted the development of antibodies against RH5 in participants’ blood, with the strongest responses observed in infants under 11 months, followed by older children and adults.
These findings are undoubtedly promising, but larger-scale studies are warranted due to the limited sample size in the current trial.
Journal article: Silk, S.E., et al. 2023. Superior antibody immunogenicity of a viral-vectored RH5 blood-stage malaria vaccine in Tanzanian infants as compared to adults. Med.
Summary by Stefan Botha