The protein GBP1 plays a critical role in our body’s natural immune response against pathogens. This protein combats bacteria and parasites by encapsulating them in a protective protein layer, though the mechanism behind this process was not fully understood until recently. Scientists have now elucidated how GBP1 functions (Figure 1).
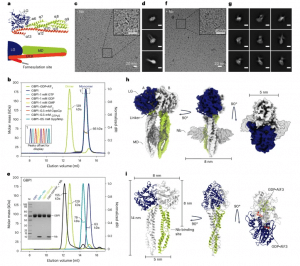
Figure 1: Cryo-EM structure of the GDP·AlF3-stabilized GBP1 dimer. a, Atomic model of monomeric GBP1 (PDB 1DG3) in cartoon representation alongside a schematic representation displaying the domain architecture. LG domain, blue; MD, green, GED, orange and red. Individual α-helices in the MD and GED are numbered sequentially. b, SEC–MALS analysis of GBP1 with different nucleotides. GBP1 appears monomeric on SEC–MALS in the presence of GTP, GDP, GMP, GMP·AlF3, GppCp, GTPyS or GppNHp, while a dimer peak emerges in the presence of GDP·AlF3. The experimentally determined molecular weight is plotted across the chromatographic peak and is reported in kDa. c,d, Representative cryo-EM micrograph (c) and 2D class averages (d) of GBP1–GDP·AlF3. The scale bar in d is 5 nm. e, SEC–MALS analysis of recombinant GBP1:Nb74 complex in the presence and absence of GDP·AlF3. An SDS–PAGE analysis of peak fractions is also shown (molecular marker in leftmost lane, in kDa). f,g, Cryo-EM micrograph (f) and 2D class averages (g) of GBP1–GDP·AlF3–Nb74 (scale bar, 5 nm). h, The 3D cryo-EM density map of the GDP·AlF3-stabilized GBP1 dimer bound to Nb74. i, Refined atomic model of the GBP1 dimer as derived from fitting into the cryo-EM density in h. The nucleotide-binding sites located at the LG dimer interface are highlighted in orange.
This discovery could pave the way for the development of new therapies and medications, particularly for individuals with weakened immune systems.
Guanylate Binding Proteins (GBPs), such as GBP1, are key players in our innate immune system. GBPs form the body’s first line of defense against various infectious diseases caused by bacteria and parasites. They also play a significant role in infections which have serious risks during pregnancy. In their recent publication they show how GBP1 proteins help the immune system combat bacterial infections.
To uncover how GBP1 performs this task, the researchers used cryo-electron microscopy to visualize the protein’s interactions with bacterial membranes at the molecular level. They captured a detailed 3D image of how the protein forms a coat around bacteria. By combining this with biophysical experiments they were able to precisely manipulate the system and decipher the antibacterial mechanism. This research deepens our understanding of how the body fights bacterial infections.
Journal article: Kuhm, T., et al., 2024. Structural basis of antimicrobial membrane coat assembly by human GBP1. Nature Structural & Molecular Biology.
Summary by Stefan Botha










