Cancer immunotherapy trains immune cells—particularly tumour-infiltrating lymphocytes (TILs)—to recognize and eliminate tumour cells. However, many cancers develop strategies to evade immune detection, leading to treatment resistance. One major factor influencing immune responses is the tumour microenvironment (TME), where cancer cells manipulate their surroundings to suppress immune activity.
A new study has uncovered a novel immune evasion mechanism in cancer, revealing how tumour cells transfer mitochondria with mutated DNA to immune cells, weakening their ability to fight cancer (Figure 1). This discovery opens the door for new treatment strategies aimed at blocking mitochondrial transfer to enhance cancer immunotherapy.
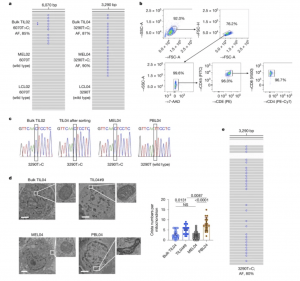
Figure 1: TILs and cancer cells share mtDNA-mutated mitochondria. a, Integrative Genomics Viewer (IGV) track data of the entire mtDNA from paired TILs and cancer cells from the same patient (02 and 04). A lymphoblastoid cell line (LCL) established from PBLs from the same patient (through Epstein–Barr virus transformation) was used as germline controls. b, Representative gating strategy for bulk TIL analyses. c, Capillary sequencing chromatograms of mtDNA from patient 04. mtDNA from sorted pure CD45+CD3+ T cells from bulk TIL04 cells, MEL04 cells and PBL04 cells were sequenced. d, Left, representative transmission electronic microscopy images of bulk TIL04, TIL04#9, MEL04 and PBL04 cells from patient 04. Right, the number of cristae per mitochondrion (n = 20 per mitochondrion) were counted and quantified. Scale bars, 2 μm. e, IGV track data of the entire mtDNA of FFPE tumour tissue from patient 04. For a and e, next-generation sequencing was used for analyses. P values (shown on the chart) were calculated using one-way analysis of variance (ANOVA) with Bonferroni correction (d). Error bars show s.e.m. AF, allele frequency; NS, not significant.
Mitochondria, often referred to as the “powerhouses of the cell,” play a crucial role in energy production and metabolic regulation. In cancer, dysfunctional mitochondria contribute to tumour growth, but their precise role in immune evasion remains poorly understood. This study is the first to demonstrate that cancer cells transfer defective mitochondria to immune cells as a means of immune suppression.
Key Findings: How Cancer Cells Transfer Mutated Mitochondria to T Cells
- Mitochondrial DNA Mutations in Immune Cells
- Analysis of TILs from cancer patients revealed that they contained the same mitochondrial DNA (mtDNA) mutations as the surrounding tumor cells.
- These mutations were associated with abnormal mitochondrial structures and dysfunction in TILs, impairing their immune activity.
- Tracking Mitochondrial Movement
- The mitochondria were transferred through:
- Tunneling nanotubes (TNTs) – direct cell-to-cell connections
- Extracellular vesicles – tiny packages released by tumor cells
- Once inside T cells, the tumor-derived mitochondria gradually replaced healthy mitochondria, leading to homoplasmy (when all mtDNA copies in a cell are identical).
- The mitochondria were transferred through:
- Impairing Immune Function
- Normally, damaged mitochondria are removed through a process called mitophagy. However, tumor-derived mitochondria resisted degradation due to co-transferred mitophagy inhibitors.
- As a result, TILs suffered from:
- Reduced cell division
- Metabolic dysfunction
- Increased oxidative stress
- Weakened immune response
- Impact on Immunotherapy Resistance
- In mouse models, TILs with dysfunctional mitochondria failed to respond to immune checkpoint inhibitors, a key type of immunotherapy.
The discovery that tumours use mitochondrial transfer as an immune suppression strategy highlights a promising new target for cancer therapy. By blocking this transfer, scientists may be able to restore immune cell function and enhance immunotherapy effectiveness.
This research provides a new perspective on tumour biology and sets the stage for more effective immunotherapies in the future.
Journal article: Hideki Ikeda, H., et al., 2025. Immune evasion through mitochondrial transfer in the tumour microenvironment. Nature.
Summary by Stefan Botha










