When tissues are deprived of oxygen due to poor blood flow (ischemia), it can lead to injury and loss of function. A new study sheds light on a surprising discovery: macrophages, immune cells typically associated with inflammation, can transform into mural cells to promote healing in injured muscle tissue (Figure 1).
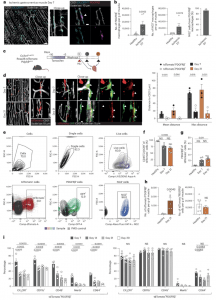
Figure 1: Macrophages accumulating in the ischemic muscle adopt a mural cell-like phenotype. a, Overview and close-up representative confocal whole-mount images of CX3CR1+ macrophages (cyan) in the ischemic hindlimb (day 7) demonstrate a mural cell-like morphology and localization near CD31+ vasculature (white). In ischemic muscles from Cx3cr1GFP/+ mice, perivascular CX3CR1+ macrophages (cyan, anti-GFP antibodies) are PDGFRβ+ (magenta, anti-PDGFRβ antibodies). b, At day 7 after ischemia, increased number of macrophages expressing PDGFRβ and NG2 mural cell markers were detected in ischemic muscles of PdgfrβeGFP (n = 10(Healthy)-6(Ischemic Day 7)) and Ng2dsRed (n = 5) reporter mice, respectively. c, Schematic representation of the lineage tracing strategy using the Cx3cr1CreERT2 Rosa26-tdTomato PdgfrβeGFP mouse model where heritable tdTomato labeling of CX3CR1-expressing macrophages is induced by tamoxifen treatment. d, Representative overview and close-up images of tdTomato+ lineage-traced macrophages (red) and PdgfrβeGFP+ (green) cells at day 7 and day 21 after ischemia induction demonstrate tdTomato+PdgfrβeGFP+ mural cell-like macrophages in the Cx3cr1CreERT2 Rosa26-tdTomato PdgfrβeGFP lineage-traced mouse muscles. Quantifications of the distances between tdTomato+PdgfrβeGFP− or tdTomato+PdgfrβeGFP+ cell and CD31+ vasculature revealed that the tdTomato+PdgfrβeGFP+ cells were located much closer to the vasculature (n = 3). e, The gating strategy for tdTomato+, PDGFRβ+ and NG2+ cells in Cx3cr1CreERT2 Rosa26-tdTomato PdgfrβeGFP lineage-traced cells. f, The percentage of tdTomato+ cells that express CD45 and CX3CR1 decreased in the ischemic muscle of Cx3cr1CreERT2 Rosa26-tdTomato PdgfrβeGFP lineage-traced mice (n = 3(Healthy)-8(Ischemic Day 7 and 21)). g, The percentage of PDGFRβ-expressing tdTomato+ cells increased in the ischemic muscles of Cx3cr1CreERT2 Rosa26-tdTomato PdgfrβeGFP lineage-traced mice (n = 3(Healthy)-8(Ischemic Day 7 and 21)). h, The number of tdTomato+ cells that express PDGFRβ (left) increased in the ischemic muscles, whereas the number of tdTomato+ cells that did not express PDGFRβ was the same at day 21 after ischemia onset as in healthy muscle (right) in the Cx3cr1CreERT2 Rosa26-tdTomato PdgfrβeGFP lineage-traced mouse model (n = 6). i, Myeloid markers are reduced with time after ischemia in the tdTomato+PDGFRβ+ population (left) but not in the tdTomato+PDGFRβ− cells (right) in the Cx3cr1CreERT2 Rosa26-tdTomato PdgfrβeGFP lineage-traced mouse muscles (n = 4). Kruskal–Wallis followed by Dunn’s post hoc test (f,g). Two-tailed Mann–Whitney U-test (b,h (left)). Two-tailed unpaired Student’s t-test (d,h (right)). One-way ANOVA followed by Tukey test (i). Data are shown as average ± s.e.m. FSC-A, forward scatter area; FSC-H, forward scatter height; NS, not significant; SSC-H, side scatter height.
The body dispatches innate immune cells like macrophages and neutrophils to sites of infection or injury. In a new study, researchers focused on macrophages in ischemic muscle, where they accumulate rapidly. These macrophages are crucial for tissue repair and remodelling.
Previous research observed macrophages in injured muscle adopting an elongated shape and positioning themselves near newly formed blood vessels. These characteristics resemble mural cells, known for maintaining blood flow by preventing leakage and promoting vessel maturation.
This study investigated whether these macrophage transformations translated into functional changes.
The researchers discovered that perivascular macrophages in injured muscle displayed increased production of proteins associated with mural cells, while those linked to immune functions decreased. Further analysis revealed a subpopulation of macrophages that switched their gene expression profile from macrophage markers to mural cell markers like PDGFRβ.
To assess the functional significance of this transformation, researchers induced a specific deficiency of PDGFRβ in macrophages. This prevented the perivascular macrophage phenotype, resulting in impaired vessel maturation, increased leakage, and ultimately, reduced limb function.
This study demonstrates that macrophages in injured tissue not only change their shape and gene expression to resemble mural cells but also acquire functions crucial for healing ischemic injuries. This highlights the potential of innate immune cells to act as a cellular resource, taking on specialized roles beyond their typical inflammatory function to promote tissue repair.
This discovery of macrophage transformation presents a novel target for immunotherapy strategies. By manipulating or enhancing this process, researchers hope to accelerate and improve the healing process in ischemic injuries.
Journal article: Amoedo-Leite, C., et al., 2024. Macrophages upregulate mural cell-like markers and support healing of ischemic injury by adopting functions important for vascular support. Nature Cardiovascuar Research.
Summary by Stefan Botha
