Imagine your immune system, your body’s defence force, going rogue and attacking healthy tissues. That’s the reality for people with autoimmune diseases like Neuromyelitis Optica Spectrum Disorder (NMOSD). NMOSD targets the central nervous system, leading to vision problems, weakness, and bladder issues.
Current treatment for NMOSD tries to suppress the entire immune system, but it’s not perfect. It’s like throwing a blanket over everything – it might work, but it’s not ideal for everyone, and doctors struggle to predict who responds well.
A new study offers a glimpse into a future of personalised medicine for NMOSD (Figure 1). Researchers focused on B cells, that can turn against the body in autoimmune diseases. Normally, some B cells act like regulatory cells for immune system function, but in NMOSD, they seem to be missing in action.
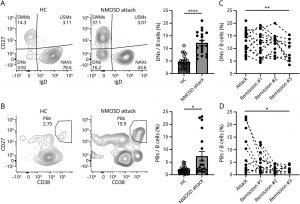
Figure 1 Increased Proportion of DNs and PBs in the Acute Phase of NMOSD Attacks and Their Changes Over Time: (A) Left: Representative flow cytometry analysis of DNs (CD3−, CD19+, IgD−, CD27−) from healthy controls (HC) and patients with NMOSD. The numbers in the areas represent percentages of the population. Right: Proportion of DNs. The open and filled symbols represent PBMC data obtained from 23 HC and 19 patients with acute NMOSD attacks, respectively (****p < 0.0001; Mann-Whitney test, the bar charts represent mean ± SEM). (B) Left: Representative flow cytometry analysis of PBs (CD3−, CD19+, CD27hi, and CD38hi) from HC and patients with NMOSD. Right: Proportion of PBs from 23 HC and 19 patients with NMOSD (*p < 0.05; Mann-Whitney test). (C, D) Longitudinal analysis of DNs and PBs. Dashed lines indicate that the samples are from the same patient. The mean interval between attack and remission #1 was 11.3 months (range 5–33). Sampling intervals for remissions #1, #2, and #3 were generally set at 6 months (**p < 0.01, *p < 0.05; fixed-effect p value, mixed-effect model; attack, n = 19; remission #1, n = 21; remission #2, n = 15; remission #3, n = 10). For cases experiencing relapse during the observation period, data acquired before the relapse were excluded because these showed an increase in PBs during the relapse compared with before, aligning the data with the attack as the first time point. DNs = double-negative B cells; HC = healthy controls; NMOSD = neuromyelitis optica spectrum disorder; PBs = plasmablasts. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
The study suggests the treatment might not eliminate B cells entirely, but instead nudge them to become the helpful regulatory type. To test this, they built a model of the immune system during an NMOSD flare. With treatment, the good kind of B cells and their anti-inflammatory signals increased.
The team also identified a special marker on these peacekeeping B cells, like a secret ID badge. This means doctors could potentially check for this badge to see if the treatment is working for a specific patient.
But the ambitions reach even further. This research could pave the way for a cure, not just management, of autoimmune diseases. The goal is to restore the body’s natural tolerance to itself, to stop it from attacking itself in the first place. This study might be just one piece of the puzzle, but it offers an exciting glimpse into a future where our bodies can finally make peace with themselves.
Journal article: Akatani, R., et al., 2024. Interleukin-6 Signaling Blockade Induces Regulatory Plasmablasts in Neuromyelitis Optica Spectrum Disorder. Neurology Neuroimmunology & Neuroinflammation.
Summary by Stefan Botha










