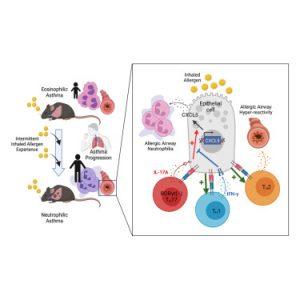
A recent study sheds light on the immune mechanisms driving neutrophilic asthma and introduces one of the first mouse models designed to mimic the condition in humans (figure 1). Their findings provide crucial insights into inflammation pathways, offering potential targets for future treatments.
While advancements in asthma treatment, particularly biologic therapies, have revolutionized care for eosinophilic asthma, neutrophilic asthma remains difficult to treat and resistant to standard therapies. This adult-onset subtype often leads to more severe disease and a lower quality of life due to persistent inflammation in the lungs.
To replicate the immune activity seen in adult lungs, researchers exposed mice to repeated, brief exposures of an inhaled allergen over time. This approach effectively mimicked airway inflammation observed in human neutrophilic asthma.
Key findings included:
- Increased CD4+ TRM Cells: The exposure triggered the accumulation of CD4+ tissue-resident memory (TRM) cells—immune cells known for their rapid response to previously encountered allergens.
- IL-17A and Neutrophil Recruitment: Upon activation, a subset of CD4+ TRM cells produced IL-17A, a pro-inflammatory cytokine that signaled lung epithelial cells to recruit neutrophils, leading to inflammation and lung damage.
While neutrophils are essential for pathogen defense, their overactivation in asthmatics causes harmful inflammation when exposed to harmless allergens.
Interestingly, the team discovered that lung epithelial cells also play a protective role by using the immune-facing molecule MHC-II to suppress inflammation.
These findings reveal key immune pathways that could be targeted to manage neutrophilic asthma. By modulating the balance between pro-inflammatory IL-17A and anti-inflammatory IFN-gamma, researchers hope to develop new therapies aimed at:
- Blocking IL-17A signaling to prevent neutrophil recruitment.
- Enhancing MHC-II pathways to boost IFN-gamma production and suppress inflammation.
- Targeting CD4+ TRM cells to modulate immune memory in the airways.
This study not only clarifies the immune drivers of the condition but also highlights potential therapeutic targets to develop precise, personalized interventions.
With further research and clinical translation, these discoveries could improve outcomes for neutrophilic asthma patients, offering hope for better disease control and quality of life.
Journal article: Raaj. V., et al. 2025. Lung CD4+ resident memory T cells use airway secretory cells to stimulate and regulate onset of allergic airway neutrophilic disease. Cell Reports.
Summary by Stefan Botha