A new study has revealed compelling evidence of blood-brain barrier dysfunction and neuroinflammation in individuals with Down Syndrome Regression Disorder (DSRD) (Figure 1). The research marks a significant step forward in understanding the biological basis of this rare and debilitating condition.
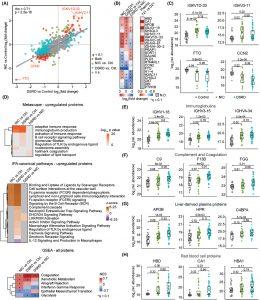
Figure 1: Proteomic dysregulation in individuals with Down Syndrome Regression Disorder (DSRD) shows similarities to neuroinflammatory conditions. (A) Scatter plot showing the correlation of fold changes of proteins in the cerebrospinal fluid (CSF) between individuals with Down Syndrome Regression Disorder (DSRD, n = 34) versus neurotypical controls (Control, n = 22) and neuroinflammatory controls (NIC, n = 27) versus neurotypical controls. The gray dotted line represents the diagonal, and the blue line represents the linear fit with 95% confidence intervals in gray. Proteins with significant (q < 0.1) fold change in both analyses are shown in red, significant in only NIC versus Control shown in green, significant in only DSRD versus Control shown in blue, and those not significant in either of the comparisons are shown in gray. Spearman correlation coefficient (rho) and p-value are provided in the top left corner. (B) Heatmaps highlighting the proteins with the top 5 strongest positive and negative fold changes in pairwise comparisons among the three sample groups (left), and their fold changes in the analysis of the plasma proteome of individuals with trisomy 21 (T21, n = 25) versus euploid controls (D21, n = 25) (right) using linear models. Asterisks indicate significant fold changes (q < 0.1) after Benjamini-Hochberg adjustment for multiple hypothesis testing. (C) Sina plots showing relative abundances of IGKV1D-33, IGHV3-11, FTO, and CCN2 in Controls (n = 22, gray), NIC (n = 27, green), and DSRD (n = 34, blue). Statistics above lines between sample groups represent q-values derived from linear models and adjusted using the Benjamini-Hochberg method. Boxes represent interquartile ranges and medians, with notches approximating 95% confidence intervals. (D) Heatmaps showing pathways and hallmarks enriched among proteins with positive fold changes in all pairwise comparisons using Metascape and IPA and analysis of all proteins ranked by log2(fold change) multiplied by −log10(p-value) in Hallmark Gene Set Enrichment Analysis. Asterisks indicate significant enrichment (q < 0.1) after Benjamini-Hochberg adjustment. E-H Sina plots showing relative abundances of proteins from select pathways: immunoglobulins IGHV1-18, IGHV3-15, and IGHV4-34 (E), complement and coagulation proteins C9, F13B, and FGG (F), liver-derived plasma proteins APOB, HPR, and C4BPA (G), and red blood cell proteins HBD, CA1, and HBA1 (H). q-values displayed above lines between sample groups are derived from linear models and adjusted using the Benjamini-Hochberg method. Boxes represent interquartile ranges and medians, with notches approximating 95% confidence intervals.
DSRD is a poorly understood but increasingly recognized condition that causes sudden and severe regression in high-functioning young individuals with Down syndrome. Previously independent individuals can abruptly lose the ability to speak, walk, feed themselves, and perform basic daily tasks. Some develop immobility or catatonia.
In this study, researchers dug deeper into the biological mechanisms behind DSRD.
One of the most striking findings from the new research is evidence of dysfunction in the blood-brain barrier (BBB). This protective barrier typically prevents harmful substances and immune cells from entering the brain. If the BBB becomes leaky, the immune system can mistakenly attack brain tissue, leading to neurological disease.
The research team collected cerebrospinal fluid (CSF) samples from three groups:
- Patients with DSRD.
- Patients with known neuroinflammatory conditions (like multiple sclerosis and autoimmune encephalitis).
- Neurotypical control patients.
By analysing these samples using proteomics (study of proteins), metabolomics (study of metabolites), and immune marker profiling, the researchers were able to identify distinct molecular patterns.
What They Found
- Upregulated Immunoglobulin Sequences: Strong indicators of neuroinflammation were present in DSRD patients’ CSF, similar to patterns seen in neuroinflammatory diseases.
- Increased Erythrocyte and Plasma Proteins: These proteins, often present when the blood-brain barrier is compromised, were elevated in DSRD patients, pointing to BBB dysfunction.
- Immune Marker Profiles: DSRD patients shared immune signatures with other neuroimmunological disorders, reinforcing the idea that DSRD is an immune-mediated condition.
These findings offer hope for targeted treatments. Understanding that DSRD is linked to neuroinflammation and BBB dysfunction opens new avenues for precision immunotherapy.
The implications of this study extend beyond DSRD. By better understanding how the immune system and blood-brain barrier interact in neurodevelopmental disorders, scientists may uncover new insights applicable to other neuroimmune conditions, including Alzheimer’s disease and autoimmune encephalitis.
Journal article: Santoro, J. D., et al. 2025. Evidence of blood–brain barrier dysfunction and CSF immunoglobulin synthesis in Down Syndrome Regression Disorder. Annals of Clinical and Translational Neurology.
Summary by Stefan Botha










