Lung infections are a major challenge, and often contribute to mortality. Innate cells, such as neutrophils are among the first cells recruited to tissues following infection, allowing them to respond rapidly to the invading pathogen. These cells have a diverse range of effector functions including phagocytosis, cytokine production as well as enclosing the pathogen within neutrophil extracellular traps (NETs). Even though these cells play an important role in immune response, their functioning is still poorly understood, as previous research has also shown that their prolonged activation can lead to excessive inflammation which negatively impacts the host, in a diverse range of disease states.
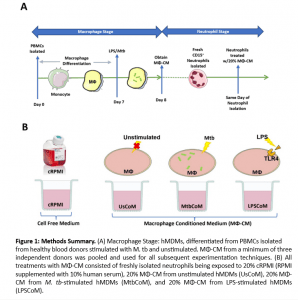
During the innate immune response, macrophages are among the first cells to encounter pathogens in the lung (for example in the case of pneumonia and M. tb infection), and recruit neutrophils to the site of infection. The neutrophils subsequently phagocytose the bacteria, while producing cytokines and chemokines for recruitment of more cells to aid in the disease control. In the present study (Figure 1), the authors examined the macrophage–neutrophil axis by assessing the effects of macrophage conditioned medium (MΦ-CM) from primary human monocyte-derived macrophages (hMDMs) stimulated with LPS or a whole bacterium (Mycobacterium tuberculosis) on neutrophil function.
Firstly, the authors noted that complementary testing to test whether MΦ-CM could activate neutrophils showed that this was achieved, as seen through the expression of CD63. They noted how neutrophils exposed to MtbCoM or LPSCoM exhibited phenotypic differences in cell morphology compared to neutrophils exposed to cRPMI and UsCoM controls, as the neutrophils had the typical elongated shaped and NETs associated with activation.
Next, they examined whether MΦ-CM could alter key effector functions of neutrophils and noted that this treatment of cells did indeed impact the ability of neutrophils to secrete certain cytokines. Increased levels of CXCL8, IL-13 and IL-6 were observed, and further flow cytometry analysis indicated that cellular pathways, specifically for ROS production, glucose uptake and apoptotic activity were altered.
Taken together, the findings highlighted in the study show that MΦ-CM from M.tb- or LPS-stimulated hMDMs (i) activates neutrophils, (ii) enhances their energetics, (iii) influences their effector function, and (iv) obstructs their ability to migrate away from the infection site. Thus, further understanding of how the microenvironment alters neutrophils (following inflammation), could aid in developing new therapies for infectious and inflammatory diseases.
Journal article: Murphy et al., 2024. Human Macrophages Activate Bystander Neutrophils’ Metabolism and Effector Functions When Challenged with Mycobacterium tuberculosis. International Journal of Molecular Sciences.
Summary by Mbali Mkhonza